Draw The Electron Configuration For A Neutral Atom Of Manganese

Imagine you've got a bunch of tiny, energetic dancers – these are our electrons. They're not just randomly flitting about; they have very specific places to be, like seats in a theater. For a neutral atom of Manganese, which we'll call "Manny" for fun, we need to figure out where all 25 of its electron dancers will settle down.
Manny the Manganese atom is like a friendly host, making sure everyone has a spot. A neutral atom means the number of positive protons in its center (the nucleus) perfectly matches the number of negative electrons buzzing around. So, Manny has 25 protons, and therefore, 25 electron dancers to place.
These electron dancers don't just pile into any old space. They have preferred "rooms" or "energy levels" where they like to hang out. Think of it like different floors in a building, with the lowest floors being the most popular and the highest floors being a bit more exclusive.
The first and lowest energy level, called the 1s orbital, is a cozy little spot. It can only hold a maximum of 2 electrons. So, our first two dancers, let's call them Pip and Pop, enthusiastically claim these prime seats. They're the first ones to arrive at the party!
Next up is the second energy level. This level has a few different types of rooms. There's another s orbital here, the 2s orbital, which is just like the 1s but a bit further out. Pip and Pop's friends, Squeaky and Squawk, happily take these two spots. They like being close, but not too close to the nucleus.
Now, this second energy level also has a set of three rooms called p orbitals. These are like little lounges. Each p orbital can hold up to 2 electrons. So, for the 2p level, we have three lounges, meaning a total of 6 more spots available.
This is where things get a little more social. Electrons in the same subshell, like these 2p lounges, tend to spread out before they start pairing up. It's like they want their own space at the lounge table before having to sit next to someone. So, if we have 6 electrons to fill these 2p lounges, one electron will go into each lounge first, and then the remaining ones will pair up.
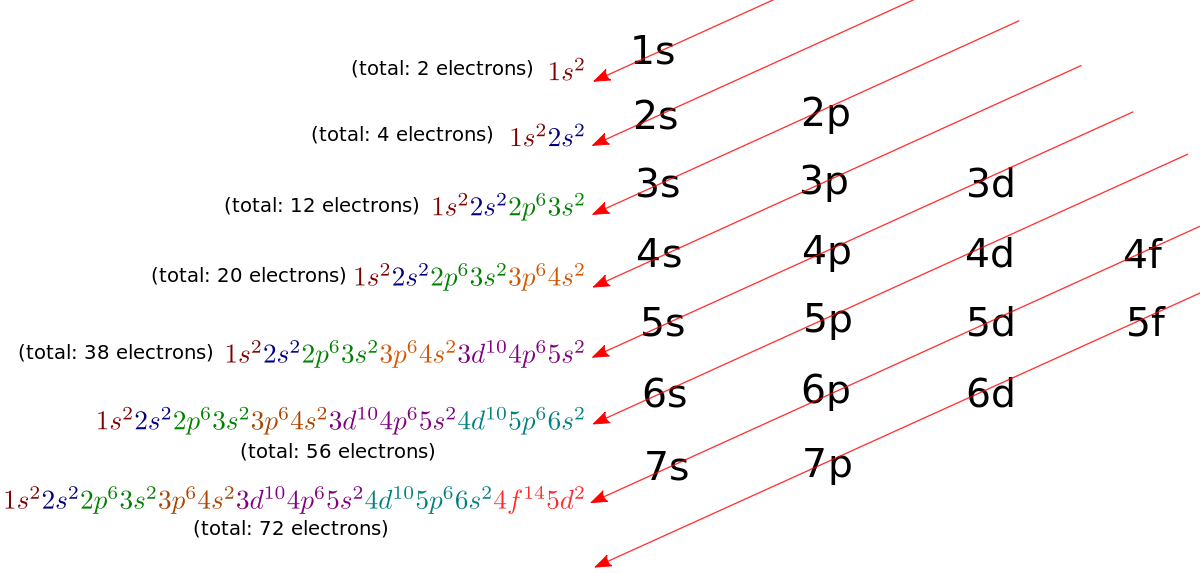
So, our electron dancers Zip, Zap, Zing, Zong, Zaz, and Zuz will fill up the 2p orbitals. That's a total of 2 + 2 + 6 = 10 electrons so far placed for Manny.
Moving on to the third energy level, we have another 3s orbital. This is a popular spot again, and Blip and Blop are happy to take the two seats here. They enjoy the slightly more spacious feeling of this level.
The third energy level also has its own set of three 3p orbitals. Just like the 2p level, these can hold a total of 6 electrons. So, Flicker, Flash, Glimmer, Gleam, Shine, and Sparkle all find their places here, spreading out first and then pairing up.
But wait, there's more! The third energy level has a special surprise: a set of five d orbitals. These are like more elaborate suites, and they can hold up to 10 electrons each! Imagine a whole new wing of the theater opening up.
This is where things get particularly interesting for Manny. He has to fill these 3d orbitals. These d orbitals are a bit more complex in their shapes, and the electrons fill them up in a specific order, following a rule that's a bit like a zigzag pattern on a chart. Think of it as a dance choreography!
Manny has 25 electrons in total. We've already placed 2 in 1s, 2 in 2s, 6 in 2p, 2 in 3s, and 6 in 3p. That's a total of 2 + 2 + 6 + 2 + 6 = 18 electrons. We still have 25 - 18 = 7 electrons left to place.
These 7 remaining dancers are heading for the 3d orbitals. Remember, each d orbital can hold up to 2 electrons, and there are 5 of them, for a grand total of 10 possible spots. Our 7 remaining dancers will fill these up, following that zigzag pattern.
So, the electrons will fill up the five 3d orbitals one by one, and then start pairing up. This means that each of the first five 3d orbitals will get one electron, and then the remaining two electrons will pair up with two of those already placed. It's like a game of musical chairs where everyone wants their own chair first!
So, for the 3d orbitals, we have Twinkle, Shimmer, Glow, Radiant, Lustrous, Brilliant, and Dazzling. Five of them will go into separate 3d orbitals initially, and then Brilliant and Dazzling will pair up with Twinkle and Shimmer, respectively.
But here's the really cool part! Because Manny is Manganese, with its atomic number 25, something slightly unexpected happens with the very next energy level. Even though the 4s orbital is technically at a lower energy level than the 3d orbitals in some scenarios, the electrons like to fill it up before the 3d orbitals are completely full in many cases. This is a bit like a shortcut or a preferred route.
So, two electrons, let's call them Dash and Dashy, go into the 4s orbital. This orbital is like a small, comfortable waiting lounge just outside the main event of the d orbitals.
Now we've placed 2 in 1s, 2 in 2s, 6 in 2p, 2 in 3s, 6 in 3p, 2 in 4s. That's 2 + 2 + 6 + 2 + 6 + 2 = 20 electrons. We still have 5 electrons left!
These last 5 electrons are the ones that will finally fill up the remaining spots in the 3d orbitals. They will go one into each of the five 3d orbitals, making them each half-full. This half-filled state for the 3d orbitals is surprisingly stable and contributes to Manganese's unique chemical properties.
So, our final electron dancers for the 3d orbitals are Sparkle, Glint, Shine, Gleam, and Glow. They each get their own individual 3d orbital, creating a beautiful, balanced arrangement.
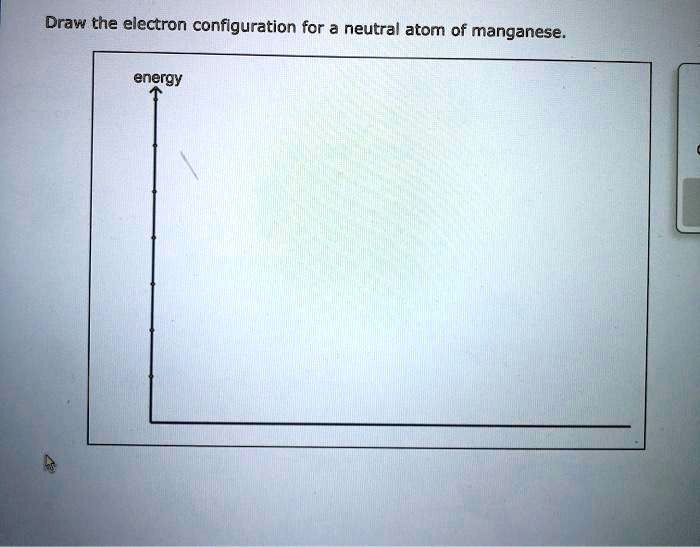
So, to recap Manny the Manganese's electron dancer configuration: We have 2 dancers in the 1s. Then 2 dancers in the 2s. Followed by 6 dancers spread across the three 2p orbitals. Next, 2 dancers in the 3s. Then 6 dancers spread across the three 3p orbitals. Then, surprisingly, 2 dancers take their seats in the 4s orbital. And finally, the last 5 dancers each take a unique spot in the five 3d orbitals.
It's like a grand performance, with each electron finding its perfect place. The configuration is written out in a shorthand notation: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵. The little superscripts tell us how many dancers are in each "room" or orbital.
It's fascinating how these tiny particles, invisible to our eyes, follow such precise rules, creating the very essence of an element like Manganese. It's a miniature universe of order and movement within every atom, a cosmic ballet happening all the time!
