Draw The Electron Configuration For A Neutral Atom Of Aluminum.
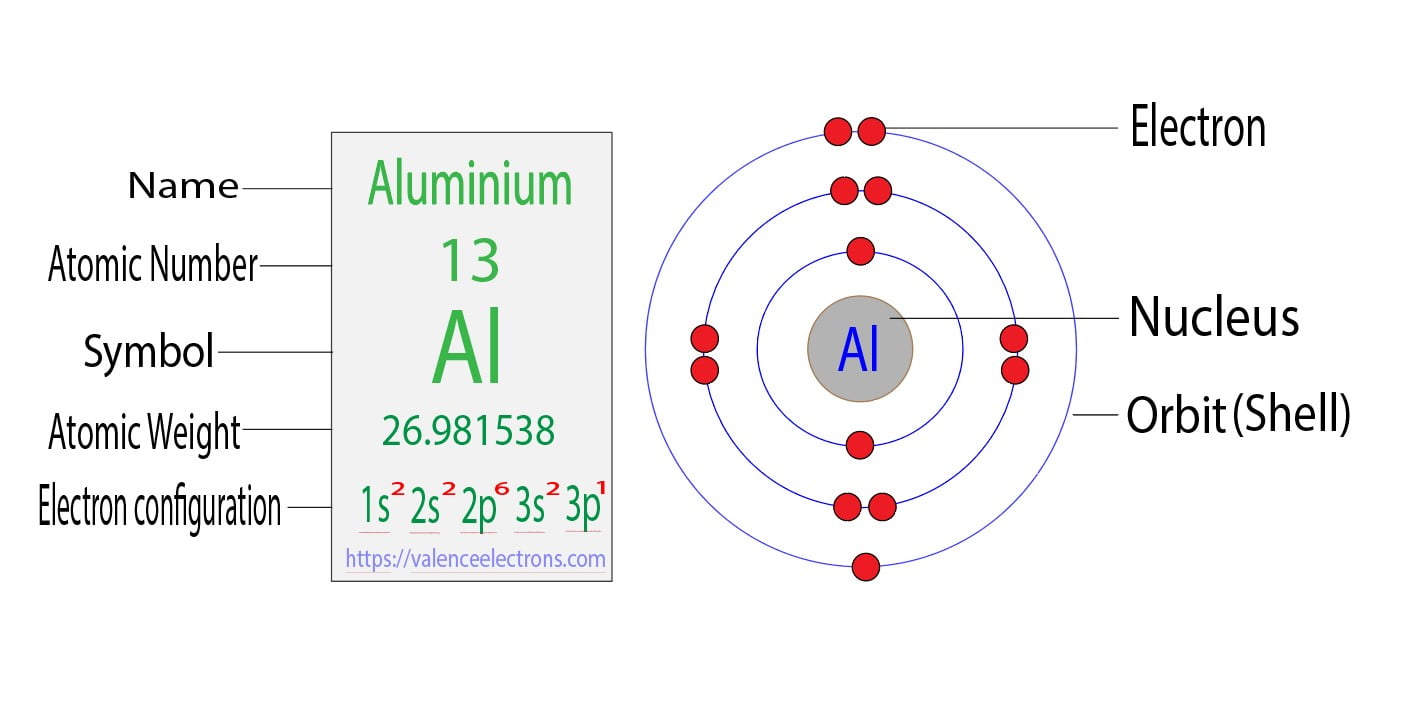
Ever found yourself staring at a blank page, a puzzle waiting to be solved, and felt a peculiar sense of satisfaction when the pieces finally click into place? That’s the joy of drawing, of visualizing, of bringing order to the seemingly complex. Today, we’re diving into a surprisingly engaging activity that might sound a little intimidating at first, but trust us, it’s a fascinating way to understand the tiny building blocks of our universe: drawing the electron configuration for a neutral atom of aluminum.
Now, you might be thinking, "Electron configuration? Isn't that for super-smart scientists in lab coats?" While scientists certainly use it, understanding this concept can offer some surprising benefits for your everyday life. Think of it like understanding how a recipe works. You don't need to be a master chef to appreciate why certain ingredients go together to make something delicious. Similarly, knowing how electrons are arranged in an atom helps us understand why certain elements behave the way they do, which in turn explains many phenomena we encounter daily. It’s the fundamental ‘how’ behind how things interact, from the conductivity of your phone charger to the way water forms ice. It’s a peek into the fundamental rules of chemistry, which, surprisingly, affects everything around you.
So, where do we see the results of this atomic arrangement in action? Aluminum itself is a fantastic example! That lightweight, shiny metal in your soda cans? That’s aluminum, and its electron configuration dictates its properties, like its ability to be shaped and its resistance to corrosion. Ever used aluminum foil? Again, that's our friend aluminum at work. The very existence of materials like stainless steel, plastics, and even the pigments in your paints can be traced back to the electron configurations of the atoms that make them up. It’s a subtle, invisible force shaping the material world we inhabit.
Ready to give this a try and unlock a new level of understanding? Here are some practical tips to make drawing the electron configuration for a neutral atom of aluminum not just informative, but genuinely enjoyable. First, gather your tools. You’ll need a periodic table – your best friend for this task! Think of it as a map. Then, a pencil and paper are all you need for the drawing itself. Next, understand the basics. We’re looking for the atomic number of aluminum, which tells us the number of protons and, in a neutral atom, the number of electrons. Then, we’ll fill up energy shells and subshells in a specific order. Don’t get bogged down in jargon; focus on the pattern. Think of it like filling seats in a stadium – there are rules about where people sit! Finally, and perhaps most importantly, embrace the process. It’s not about perfection on the first try. It’s about the discovery, the visual unfolding of how an atom is structured. Celebrate each correctly placed electron. You're literally drawing the blueprint of matter!
By understanding the electron configuration of aluminum, you’re not just learning a science concept; you’re gaining a deeper appreciation for the intricate, elegant design of the world around you. So, grab your pencil, consult your periodic table, and get ready to draw your way to a better understanding of the universe!
