Draw The Correct Bond Line Structure For The Following Compound
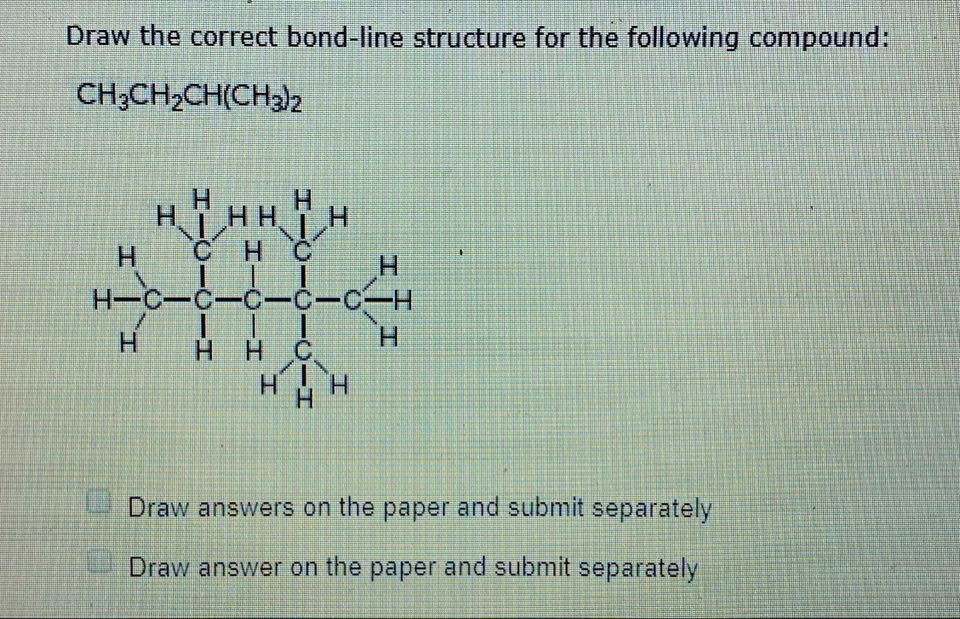
Ah, the joys of chemistry. Specifically, the thrilling world of drawing bond-line structures. Some people find it a breeze, a delightful little puzzle. Me? I find it... well, let's just say my relationship with it is complicated. Like that one sock that always goes missing in the laundry.
We're about to embark on a grand adventure, a quest to draw the correct bond-line structure for a certain compound. No biggie, right? Just a few lines, a few angles, a sprinkle of genius. Easy peasy, lemon squeezy. Except sometimes it feels more like wrestling an octopus in a bathtub.
Let's face it, sometimes the simplest things can be the most infuriating. You look at the name, you picture the atoms, and then you try to translate that into those neat little zig-zags. It’s like trying to follow IKEA instructions for a bookshelf when you’re slightly sleep-deprived.
The goal is simple: represent a molecule using only lines for bonds and vertices for atoms (unless it's carbon, which we often just assume is there, lurking in the shadows). No pesky H's, no clunky symbols. Just pure, unadulterated molecular art. Or so they tell us.
My internal monologue usually goes something like this: "Okay, so we have a [Insert Chemical Name Here]. Sounds fancy. Probably means a lot of connections. Where does this carbon go? And this oxygen? Is this a happy little three-membered ring or a sad, stretched-out chain?"
It’s a real test of spatial reasoning, isn't it? My brain often decides to take a short vacation right at the crucial moment. It’s like, "Nope, can't do angles today. How about a nice oval instead?" Thanks, brain. Super helpful.
And don't even get me started on stereochemistry. That's when the lines start to get wobbly, and suddenly you're dealing with wedges and dashes. Suddenly, what seemed like a straightforward drawing turns into a full-blown 3D optical illusion. My eyes start to cross, and I wonder if I should just go lie down.
Let's be honest, the truly correct structure requires a level of precision that sometimes feels beyond mortal comprehension. You draw it, you squint, you compare it to the textbook picture, and then you have that nagging feeling. Is it exactly right? Or is it just… close enough?
I have an unpopular opinion, you see. I think sometimes these bond-line structures are a bit too minimalist. Like they’re bragging about how elegant they are. “Look at me,” they seem to say, “so simple, so clean.” Meanwhile, my brain is over here, sweating, trying to figure out where all the implied hydrogens have gone.
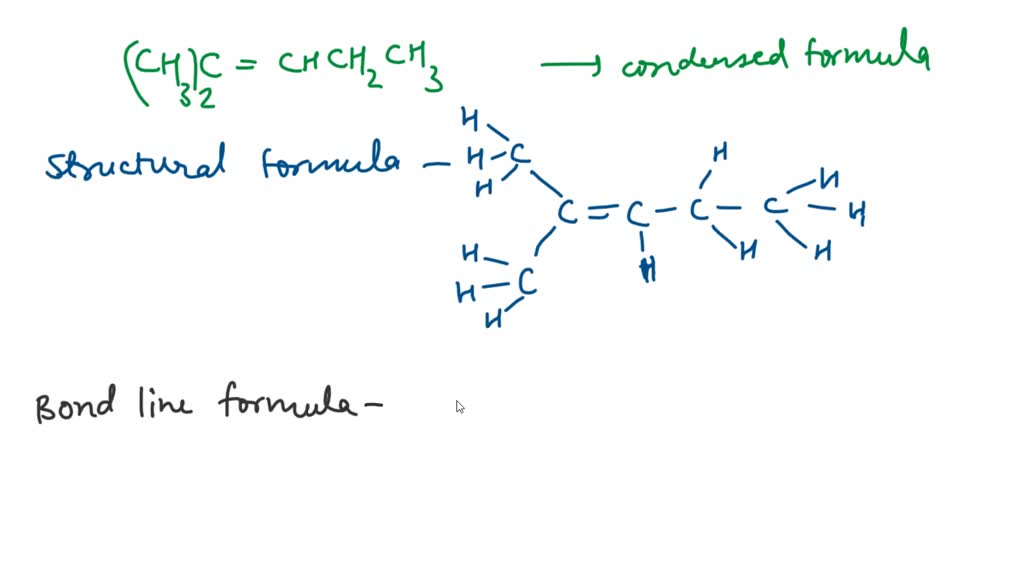
It's like being told to build a house with only a hammer and a few nails. You can do it, theoretically, but there's a certain artistry, a certain magic involved in making it not look like it's about to collapse. And that magic, my friends, is sometimes elusive.
We're looking at a specific compound today. The instructions are clear: draw the correct bond-line structure. No pressure, right? Just a little task that separates the chemistry wizards from the rest of us mere mortals who occasionally get our lone pairs mixed up.
Imagine a tiny, invisible dance party of atoms. That's what a molecule is. And we're trying to draw a map of that dance party. But the map doesn't show the dancers’ names, or their favorite dance moves. Just where they're standing and who they’re holding hands with. Metaphorically speaking, of course. Usually.
So, we have our compound. Let’s call it the Mysterious Molecule for now. Its official name is probably something that sounds like a robot trying to clear its throat. But in bond-line structure, it should be a thing of beauty. A sleek, efficient representation of atomic camaraderie.
My process often involves a lot of erasing. And then re-erasing. And then maybe drawing it on a separate piece of paper, just in case. It’s a journey of discovery, really. A journey that sometimes leads to triumphant “Aha!” moments, and other times leads to quiet sighs of resignation.
Sometimes, I suspect the chemists who invented these things were just showing off. “Oh, you draw out all the atoms and bonds? How quaint. I can do it with just lines. Watch this!” And then they proceeded to make our lives… interesting.
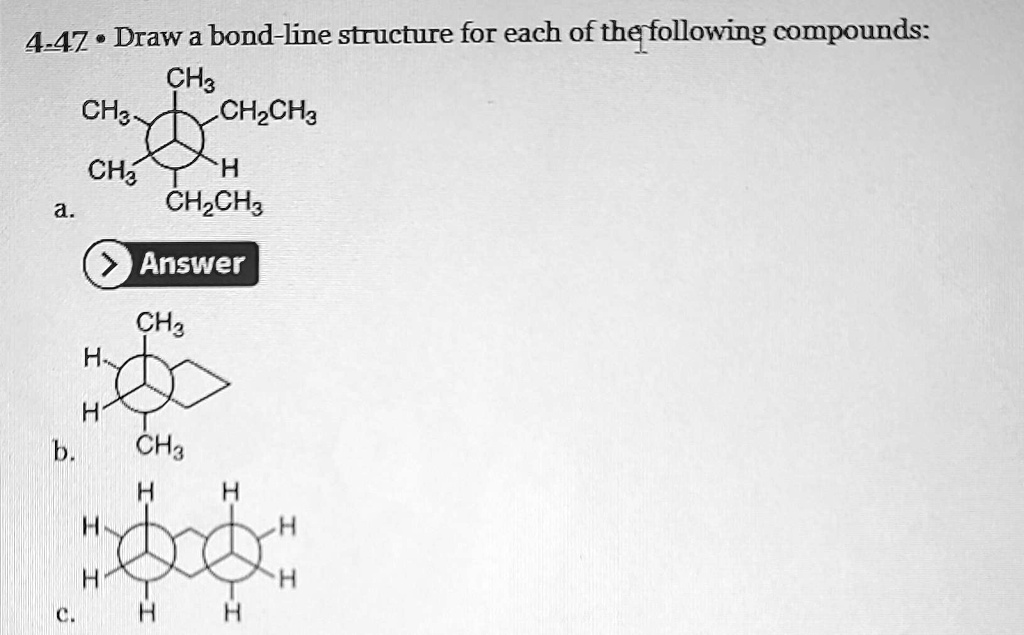
The beauty of the bond-line structure is its efficiency. It’s the molecular equivalent of a perfectly curated social media profile. Everything you need to know, presented in the most streamlined way possible. But sometimes you miss the little details, don't you?
For our Mysterious Molecule, we need to be precise. Every turn of the line, every vertex, matters. It’s like a secret code. And we’re the code-breakers. Or at least, we’re trying to be.
There are rules, of course. Rules about angles, about how many bonds each atom should have. And if you forget those rules? Well, that’s when you get a structure that’s technically a bond-line drawing, but utterly wrong. Like wearing socks with sandals. Technically a fashion choice, but….
Let’s break down the hypothetical process. First, you identify the longest carbon chain, or the principal functional group. This is like finding the main stage at the atomic dance party. Then you start attaching everything else. Think of it as adding the guest list to your event planner.
You have to remember that carbons love to make four bonds. Oxygens like two. And nitrogens? They’re usually happy with three. If your structure doesn't follow these unspoken agreements, it's like the dancers are all doing their own thing, and it's chaos.
And then there are those pesky heteroatoms – the non-carbon, non-hydrogen elements. They get to keep their symbols. So you might see an ‘O’ or an ‘N’ sticking out, like a celebrity at the party. They demand to be noticed.

The actual drawing part is a dance in itself. A delicate ballet of pencil on paper, or finger on screen. You aim for those nice, clean 120-degree angles for sp2 hybridized carbons, and the slightly more complex, tetrahedral arrangements for sp3. It’s a geometry lesson disguised as a chemistry problem.
My brain often prefers to draw a smiley face when faced with complex bond angles. It's a coping mechanism.
My personal struggle is with cyclic structures. Rings. They look so neat and tidy in the book. But when I draw them, they often end up looking more like a crumpled piece of paper. Or a slightly lopsided hexagon.
For our Mysterious Molecule, let’s imagine it has a ring. Is it a perfect pentagon? A slightly elongated hexagon? Or did I accidentally create a Mobius strip? The fate of chemical accuracy hangs in the balance.
And then there’s the matter of implied hydrogens. They’re the invisible guests at the party. They’re there, filling in the gaps to make sure everyone has the right number of friends (bonds), but we don’t draw them. It’s a minimalist aesthetic I can appreciate, but it means I’m constantly counting. "Okay, this carbon has three lines. It needs one more hydrogen. Phew."
It’s a bit like a magic trick. Poof! The hydrogens are gone! But they’re still there, contributing to the overall structure. You just have to trust that they exist. Which, in chemistry, is a big leap of faith sometimes.
The ultimate goal is a structure that is both accurate and unambiguous. If someone else looks at your drawing, they should be able to decipher the exact arrangement of atoms and bonds. No guessing games. No “is that a double bond or did the pencil slip?”
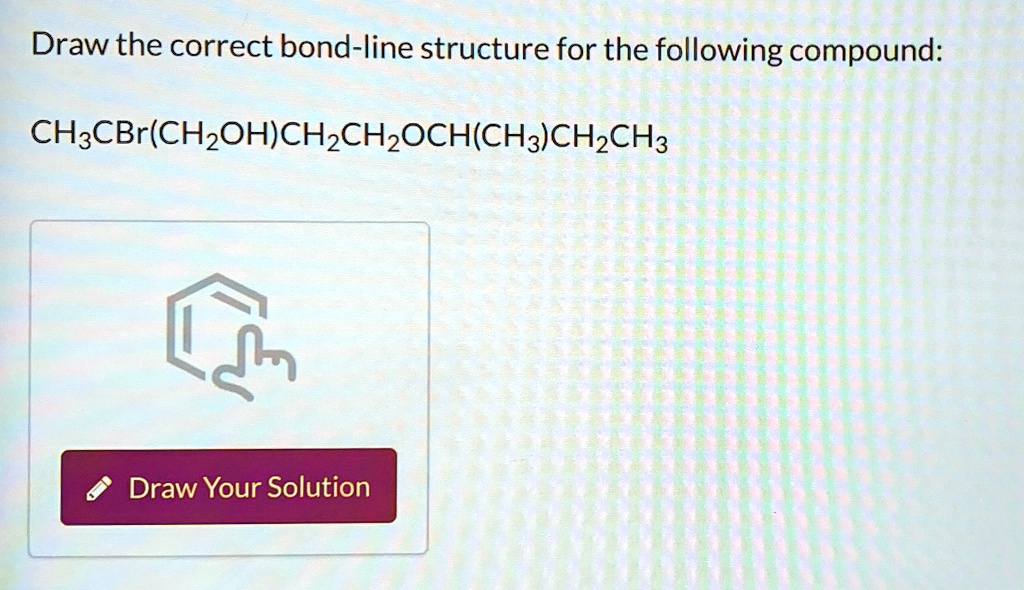
So, for this specific compound, we’re going to put on our best molecular architect hats. We’ll carefully construct it, line by line, vertex by vertex. We’ll ensure that all the implicit hydrogens are accounted for, even if they remain unseen. We'll make sure those angles are as crisp as a freshly made potato chip.
It's a challenge, for sure. But there's a certain satisfaction in getting it right. It's like solving a particularly tricky crossword puzzle. A quiet, internal victory. And maybe, just maybe, a slight nod from the universe acknowledging our efforts.
So, let's draw. Let's connect. Let's bring this Mysterious Molecule to life in its most efficient, bond-line form. And if it takes a few tries, and a moment of existential dread, well, that's just part of the fun. Isn't it?
The important thing is to not let those wiggly lines and implied carbons win. We are the masters of molecular representation. Or at least, we're learning to be. One zig-zag at a time.
And if all else fails, just remember: carbon is the best friend of the bond-line structure. It's always there, at the corners, ready to form a connection. A true team player.
Let the drawing commence! May your lines be straight (or perfectly angled) and your implied hydrogens plentiful. This is our moment to shine in the world of simplified molecular diagrams.
