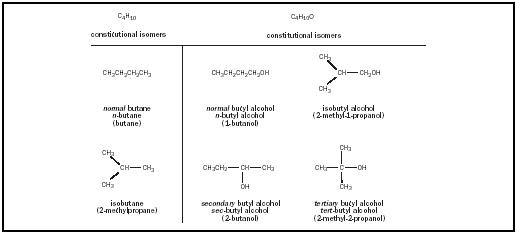
Draw Bond-line Structures For All Constitutional Isomers Of C4h10.
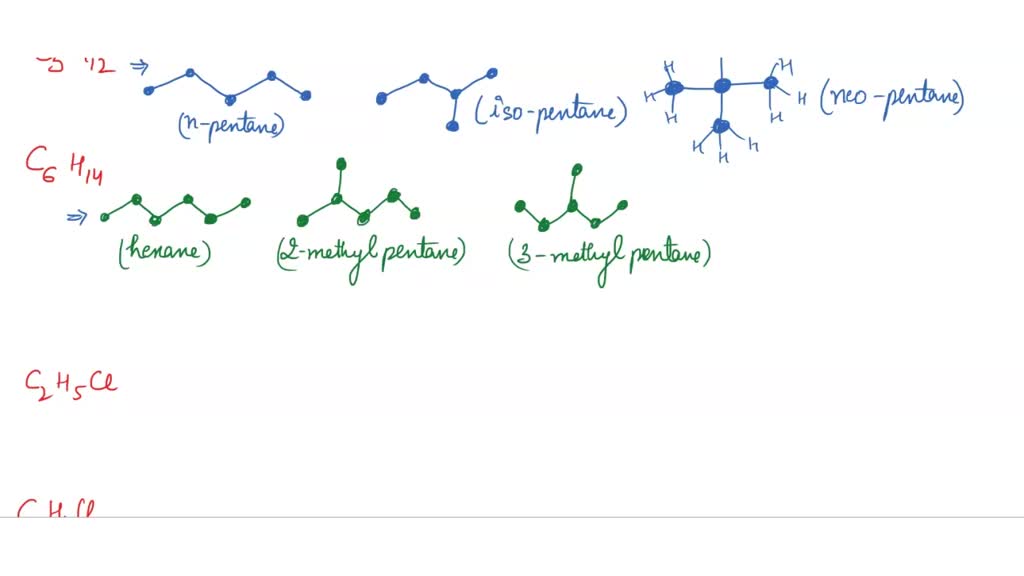
Alright, let's dive into the wonderfully quirky world of molecules. We're going to play a little game today, and it involves a very specific recipe: C4H10. That's four carbon atoms and ten hydrogen atoms, a seemingly simple combination. But oh, the mischief these atoms can get up to!
Our mission, should we choose to accept it (and we totally do, because it's fun!), is to draw all the different ways these four carbons and ten hydrogens can link up. Think of it like building with LEGOs, but with atoms. Some arrangements will look pretty straightforward, like a neat little line. Others might get a bit more… creatively tangled.
And here's a little confession: I think drawing these tiny molecular maps is secretly way cooler than most people admit. It's like solving a tiny, three-dimensional puzzle. You get to see how the same ingredients can make slightly different "dishes."
So, grab your imaginary pencil (or a real one, no judgment here!). We're about to become atom architects. Our goal is to find all the unique "blueprints" for C4H10. Let's see what kind of molecular characters we can create.
The Straight Shooter
First up, the most obvious arrangement. Imagine the four carbon atoms holding hands in a perfectly straight line. This is our anchor, our starting point. It's like the "default setting" for C4H10.
We just connect them end-to-end. C-C-C-C. Easy peasy. Now, we sprinkle in all those hydrogen atoms. Each carbon wants to be buddies with four things (either other carbons or hydrogens). So, the two end carbons get three hydrogens each. The two middle carbons get two hydrogens each. Voila!
This super-straight structure has a name, of course. It's called n-butane. The "n" stands for "normal," which, let's be honest, is code for "boring but reliable." It’s the sensible shoe of the C4H10 family.
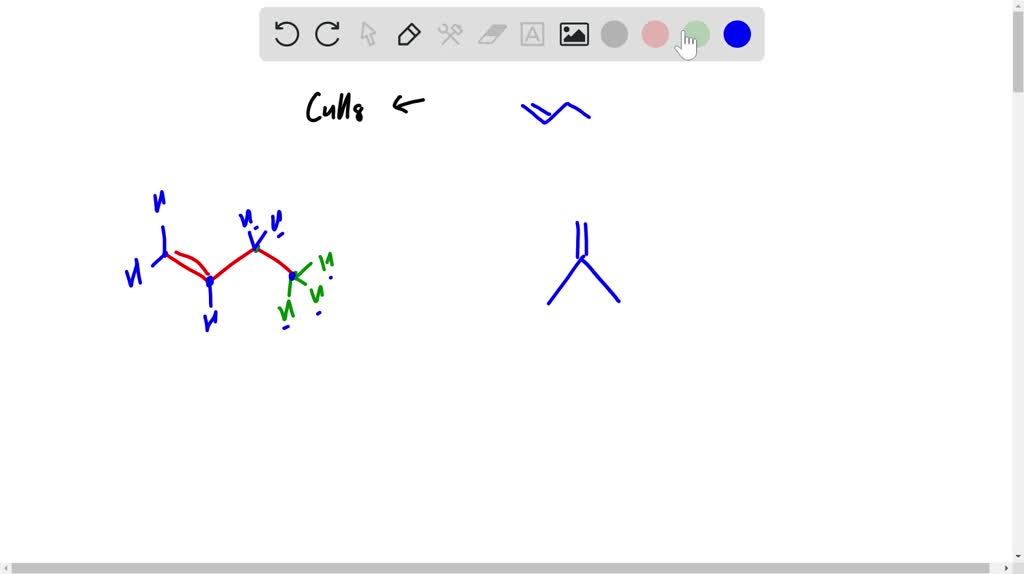
When we draw it as a bond-line structure, it's just a zig-zag line of four points. Each point is a carbon. The hydrogens are implied, happily attached to make everyone a comfortable four bonds. No need to draw every single little hydrogen. That would be, dare I say, tiresome.
So, the first isomer is our trusty n-butane. It’s the foundation upon which we’ll build our molecular wonderland. Don't underestimate the power of a straight line, folks. It's a classic for a reason.
The Branch Manager
Now, things get a little more interesting. What if we take one of those carbon atoms and move it off the main line? Instead of a perfectly straight chain, we'll introduce a little "branch."
Imagine our four carbons again. Let's make a main chain of three carbons. C-C-C. But then, we'll take the fourth carbon and attach it to the middle carbon of that three-carbon chain. It’s like our neat line decided to grow an extra arm.
This structure looks like a little "T" shape, or maybe a tiny tricycle if you squint. The carbons are still connected, just not in one long row. We still need to make sure every carbon has its four buddies. So, the carbons on the ends of the branches and the main chain get three hydrogens. The central carbon, which is connected to three other carbons, only gets one hydrogen.
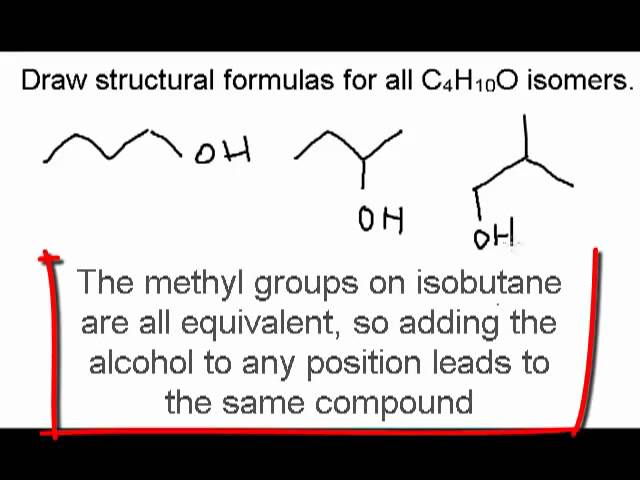
This branched fellow has a rather fitting name: isobutane. The "iso" part is like a secret handshake among certain molecular families, indicating a slight variation. It's the slightly quirky cousin to n-butane.
In bond-line structure, this looks like a three-carbon line with a shorter line sticking out from the middle carbon. Again, the hydrogens are all tucked away, doing their jobs without needing a starring role in our drawing. It's elegant, really.
So, we’ve found our second isomer: isobutane. It shows us that simply rearranging the connections can create a whole new molecule. It's proof that even simple building blocks can lead to diverse structures.
Are We There Yet?
You might be thinking, "That's it? Two isomers? We've only used four carbons!" And you'd be right to question. The beauty of these molecular puzzles is that sometimes the possibilities are limited, and sometimes they're surprisingly vast.
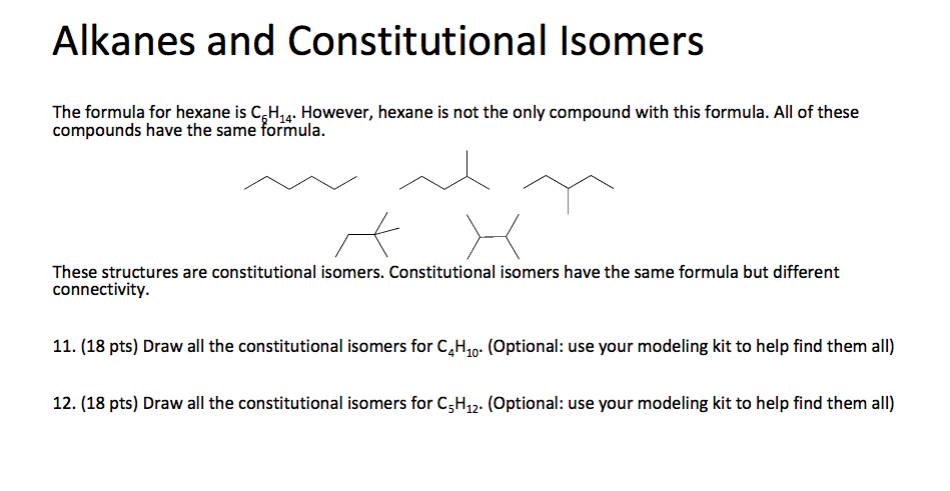
But in the case of C4H10, the universe of constitutional isomers is, shall we say, exclusive. We've explored the linear arrangement, and we've explored a single branch. What other ways can four carbons be connected?
Could we have more branches? Well, if we tried to put a branch on an end carbon of the three-carbon chain, it would just extend the main chain, making it four carbons long again. That would just lead us back to n-butane. The universe, it seems, is not keen on repeating itself with this particular set of atoms.
What about making a "Y" shape with the carbons? If you try to arrange the four carbons in a symmetrical "Y" or a star shape, you'll find that you can't actually connect them all with single bonds while keeping the "T" shape. It just doesn't work out with four carbons.
Think about it: to have more branches, you need carbons with more than two neighbors. In isobutane, we have one carbon with three neighbors. To make another isomer, you'd need to add more branching points, which would require more carbons or a different arrangement entirely.
So, it turns out that for C4H10, there are precisely two constitutional isomers. Just two! It's like a party with only two distinct dance styles.
The Unpopular Opinion
And this is where my slightly odd enthusiasm for drawing these things comes in. Many people find this kind of molecular drawing tedious. They see it as rote memorization or just scribbling lines. But I see it as unlocking secrets.
Each of these isomers, n-butane and isobutane, has its own unique personality. They have different boiling points, different melting points, and they react in slightly different ways. All from the same atomic ingredients, just arranged differently! It's like getting different flavors of ice cream from the same base ingredients. Vanilla and chocolate have a lot in common, but they are undeniably distinct experiences.
The simplicity of this example, C4H10, is actually quite profound. It highlights a fundamental concept in organic chemistry: isomerism. It shows that structure dictates properties. It's the subtle dance of atoms that creates the diversity of the world around us.
So, the next time you hear someone grumbling about drawing bond-line structures, just remember the two faces of C4H10. Remember n-butane, the straight and narrow, and isobutane, the slightly more adventurous brancher. They're a tiny, but mighty, testament to the endless possibilities hidden within the seemingly simple language of chemistry. And that, my friends, is something worth smiling about.
The two constitutional isomers of C4H10 are n-butane and isobutane.
