Draw All Reasonable Resonance Structures For The Following Species

Hey there, fellow curious minds and aspiring chemists (or just folks who like doodling electrons)! Today, we're diving into a super-duper fun topic that's like a molecular treasure hunt. We're going to be drawing all the reasonable resonance structures for a given species. Think of it as finding all the different ways a molecule can be dressed up, and it's way more exciting than picking out your outfit in the morning!
Imagine you have a really cool toy, like a LEGO spaceship. Now, you could build that spaceship in a few slightly different ways, right? Maybe you put a different antenna on it, or a couple of extra shiny bits. That's kind of what resonance is like for molecules. They're not stuck in just one static picture; they can have a few different, equally valid "poses" or "looks."
And don't you worry, this isn't some scary, complicated science-y thing. We're going to break it down so it feels as easy as drawing a smiley face. Our goal is to be super thorough, like a detective looking for every single clue!
The Grand Adventure of Electron Shuffling!
So, what does it mean to "draw all reasonable resonance structures"? Well, it means we're going to use our trusty drawing tools (a pencil and paper, or even a digital canvas!) to show how electrons can move around within a molecule. These electrons are like little nomads, always looking for a comfy spot to hang out.
When we talk about a "species," we're just talking about a specific molecule or ion. It's like saying, "Let's focus on this particular LEGO spaceship." And "reasonable" means we're not going to draw any crazy, outlandish structures that don't make any chemical sense. We're sticking to the rules of the molecular universe, which are actually pretty logical once you get the hang of them.
Think of it like a game of musical chairs, but with electrons! They hop from one place to another, creating slightly different arrangements of the molecule. Each of these arrangements is a resonance structure. And we want to find all of them that are plausible, the whole crew of possible electron configurations.
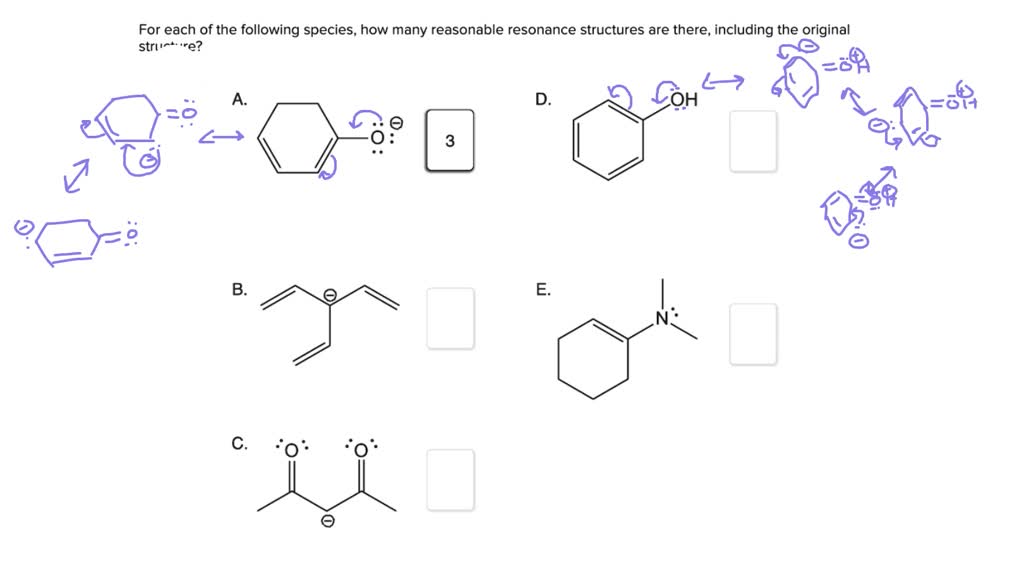
Our Marvelous Mission: Unveiling All the Looks!
The real magic is in understanding that the actual molecule isn't just one of these drawings. It's like a super-blend of all the reasonable resonance structures, an average of all its possible outfits. This is called a resonance hybrid. So, the molecule is always a little bit of this and a little bit of that, all at once! It's like having a chameleon that can be slightly purple and slightly green at the same time.
Our mission, should we choose to accept it (and we totally should because it’s awesome!), is to identify all these different, yet equally valid, ways the electrons can be distributed. We'll be looking for places where electrons can "shift" or "delocalize." It's like giving the electrons a mini-vacation to different parts of the molecule.
We're not just sketching randomly; there are some guiding principles. We're looking for things like double or triple bonds next to single bonds, or lone pairs of electrons sitting near a positive charge. These are the "invitations" for electrons to get on the move! It's like finding the red carpet that leads to a new electron party location.

Every reasonable resonance structure we draw will have the same number of atoms, and all the atoms will be connected in the same way. The only thing that changes is where the electrons are. This is super important! It means the skeleton of the molecule stays the same, but the electron "decoration" can vary.
Let’s get our detective hats on! We’re going to be on the lookout for those tiny, but mighty, electron-pushing arrows. These arrows are like little speedometers, showing us the direction and the movement of electrons. They’re the secret language of resonance, guiding us through the electron migration.
So, when you see a molecule, don't just draw one static picture. Think about all the places those electrons could be chilling. It’s like looking at a flipbook animation and drawing out each individual frame that makes the whole thing move. Each frame is a resonance structure!
And the best part? This process helps us understand why molecules behave the way they do. It explains their stability, their reactivity, and all sorts of cool chemical properties. It’s like understanding how a toy works by seeing all the different ways it can be assembled and used.
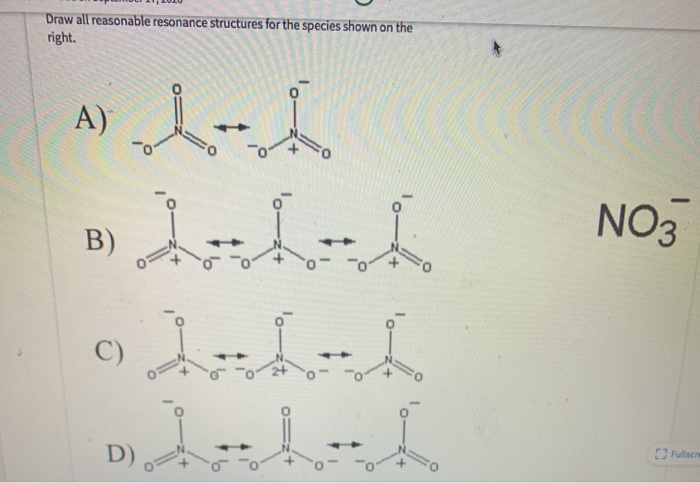
We'll be using special double-headed arrows (⇌) to show that these are resonance structures. This arrow is like a handshake between different possibilities, a signal that they are related and interchangeable in terms of electron placement. It’s the official symbol of our resonance club!
Remember, we're aiming for "reasonable." This means we're not going to create structures where atoms have way too many or way too few electrons. We’re keeping things balanced and chemically sound. Think of it as following the recipe perfectly, even when you’re making up a few variations of the cake.
So, grab your virtual tools, get excited, and let's get ready to draw! This is where chemistry gets really visual and a whole lot of fun. We’re not just memorizing facts; we’re seeing the dynamic nature of molecules. It’s like watching a dance, but with electrons!
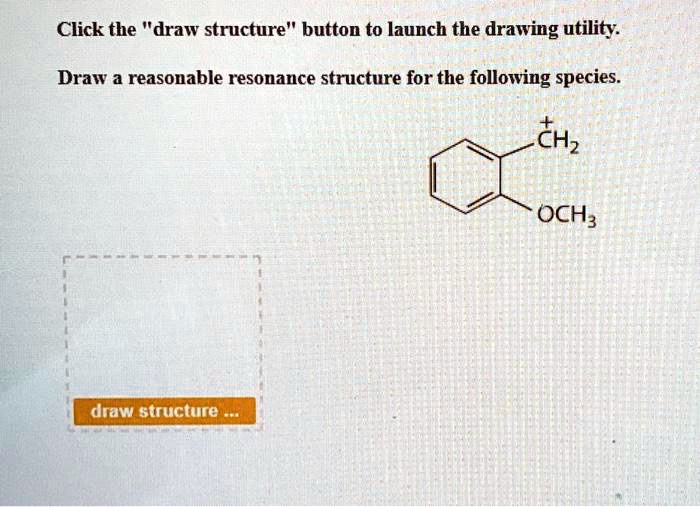
The more resonance structures we can draw for a species, the more stable it generally is. This is because the electron "blame" or "credit" is spread out, like sharing a pizza so no one person has to eat the whole thing. A delocalized electron is a happy, stable electron!
So, no pressure, just exploration. Let your inner artist and your inner scientist collaborate. We’re going to draw, we’re going to connect, and we’re going to embrace the beautiful flexibility of molecules. Get ready to be amazed by the electron shuffle!
Think of it as giving the molecule a wardrobe change, but instead of clothes, it’s rearranging its electron accessories. We want to capture every single fabulous outfit it can wear. And the more outfits, the more interesting and stable the molecule becomes!
This isn't about being perfect on the first try. It's about the process of discovery and understanding. So, let's embark on this delightful journey of drawing resonance structures, and feel that wonderful sense of accomplishment as we uncover all the reasonable possibilities!
