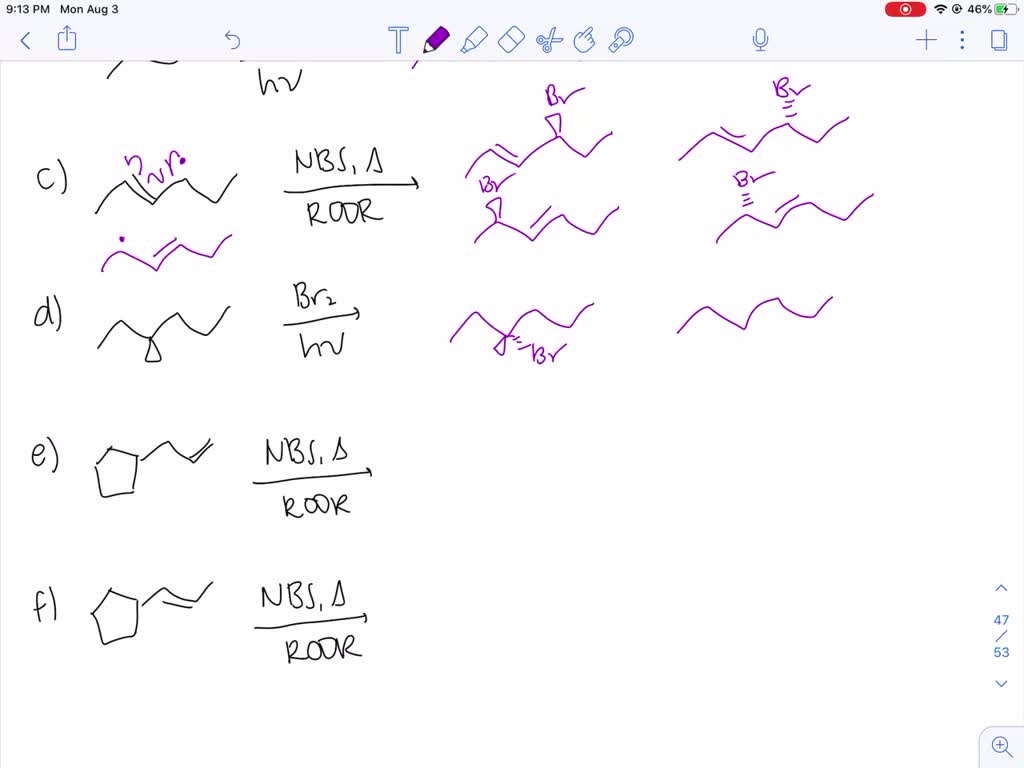
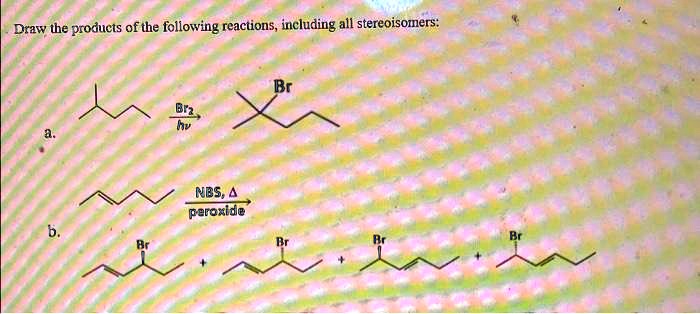
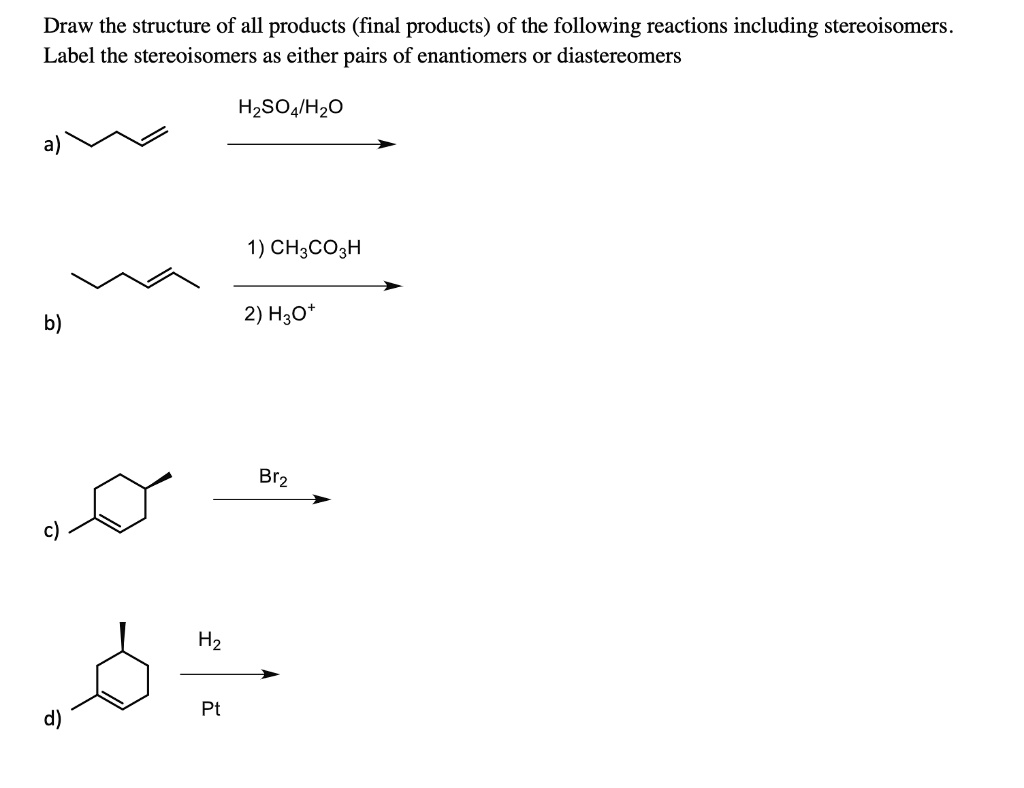
Draw All Products Including Stereoisomers In The Following Reaction
Imagine you're at a grand masquerade ball, but instead of people, it's molecules! They're all dressed up, twirling and changing costumes. Our story today is about one such molecular party, where a starting molecule, let's call it the "Grand Gatsby", decided to have a little fun and transform into something new. But here's the twist: The Grand Gatsby isn't just one entity; it's got a whole family of look-alikes, its "Mirror Image Siblings", also known as stereoisomers. They might look identical in a flat picture, but hold them up to a mirror, and poof – they're different! It’s like a pair of gloves; they’re both gloves, but you can’t wear the left one on your right hand, right?
Our Grand Gatsby molecule, when it decides to get all fancy and transform, doesn't just turn into one single thing. Oh no, it throws a whole party and invites its Mirror Image Siblings to join the transformation. Think of it like a celebrity chef deciding to make a signature dish. They don't just make one plate; they whip up several, each with a slightly different garnish or seasoning, offering a unique flavor profile. In the world of chemistry, these "flavor profiles" are the different structures these molecules can take on, and they’re called products.
Now, the challenge we're looking at is like being the official photographer at this molecular masquerade. We need to capture everyone who shows up at the party. Not just the main celebrity, but all their relatives, even the ones who are just a reflection! So, for our Grand Gatsby, when it undergoes its transformation, it’s not just going to hand over one final molecular form. It’s going to produce a whole ensemble of possibilities. We need to draw them all, every last one, including those sneaky stereoisomers that are the molecular equivalent of left-handed and right-handed scissors. They might seem the same from afar, but they have distinct "handedness," and that matters!
Let's delve a little deeper into the magic. Sometimes, molecules have a special kind of symmetry – or lack thereof – that allows them to exist in these mirror-image forms. It's a bit like how your hands are mirror images of each other. You can't perfectly superimpose your left hand onto your right hand, no matter how you try. Chemical structures can be like that too, particularly when they have a central atom (often carbon) with four different things attached to it. This is the birth of chirality, the scientific term for this "handedness." And where there's chirality, there's often a whole family of stereoisomers waiting to be discovered and drawn!
So, when our Grand Gatsby starts its reaction, it’s like a tree shedding its leaves. Not all leaves are identical, and the way they fall can vary. Some might flutter down gracefully, others might be whisked away by a gust of wind. In the chemical reaction, this translates to different pathways the molecule can take. Some pathways lead to one type of product, while others, especially those involving the chiral center, lead to its Mirror Image Sibling. And just to make things extra interesting, sometimes these reactions can produce both forms, or even favor one over the other. It’s like a choose-your-own-adventure book, but with atoms!
The truly heartwarming part of this is that each of these molecular creations, each product and each stereoisomer, has its own unique role and function. Think about how your body uses different molecules for different tasks – one for energy, another for building tissues. Even slight differences, like the "handedness" of a stereoisomer, can mean the difference between a medicine that heals and one that doesn't work, or even causes harm. It’s a delicate dance of molecular architecture, and we, as observers, are tasked with sketching every single dancer and their mirrored twin. It's about appreciating the full diversity of the molecular world, the predictable and the delightfully surprising.
So, when you see a reaction and are asked to draw all the products, including the stereoisomers, don't be intimidated! Think of it as a treasure hunt. You're not just looking for one gold coin; you're looking for all the coins, plus their reflections! It's a chance to appreciate the subtle beauty and complexity that molecules possess, and to understand that even the smallest differences can lead to a whole new world of possibilities. It's a reminder that nature, in its infinite wisdom, loves variety, and we're invited to be its enthusiastic catalogers, drawing every last, unique, and sometimes, hilariously mirrored, creation.
