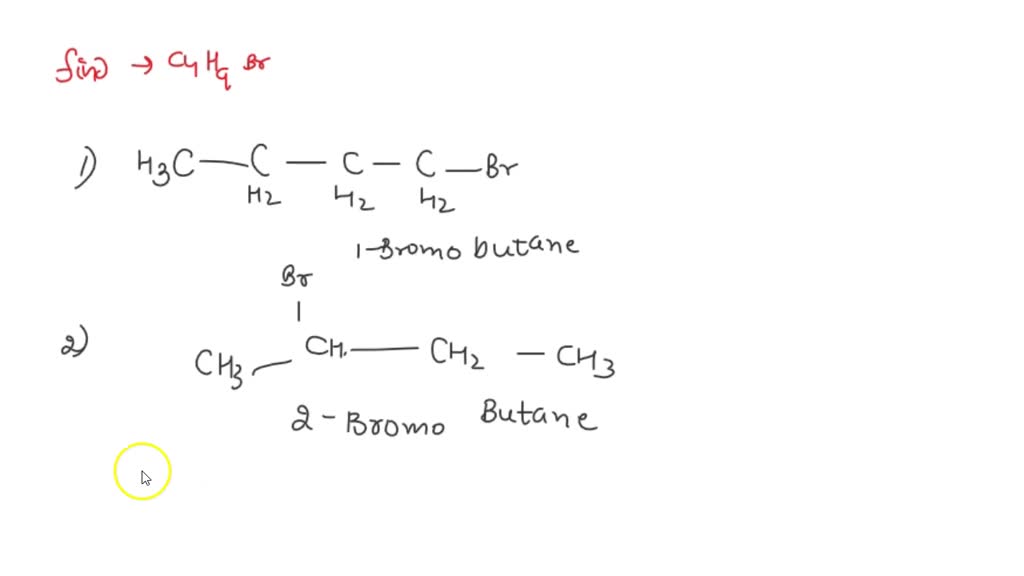
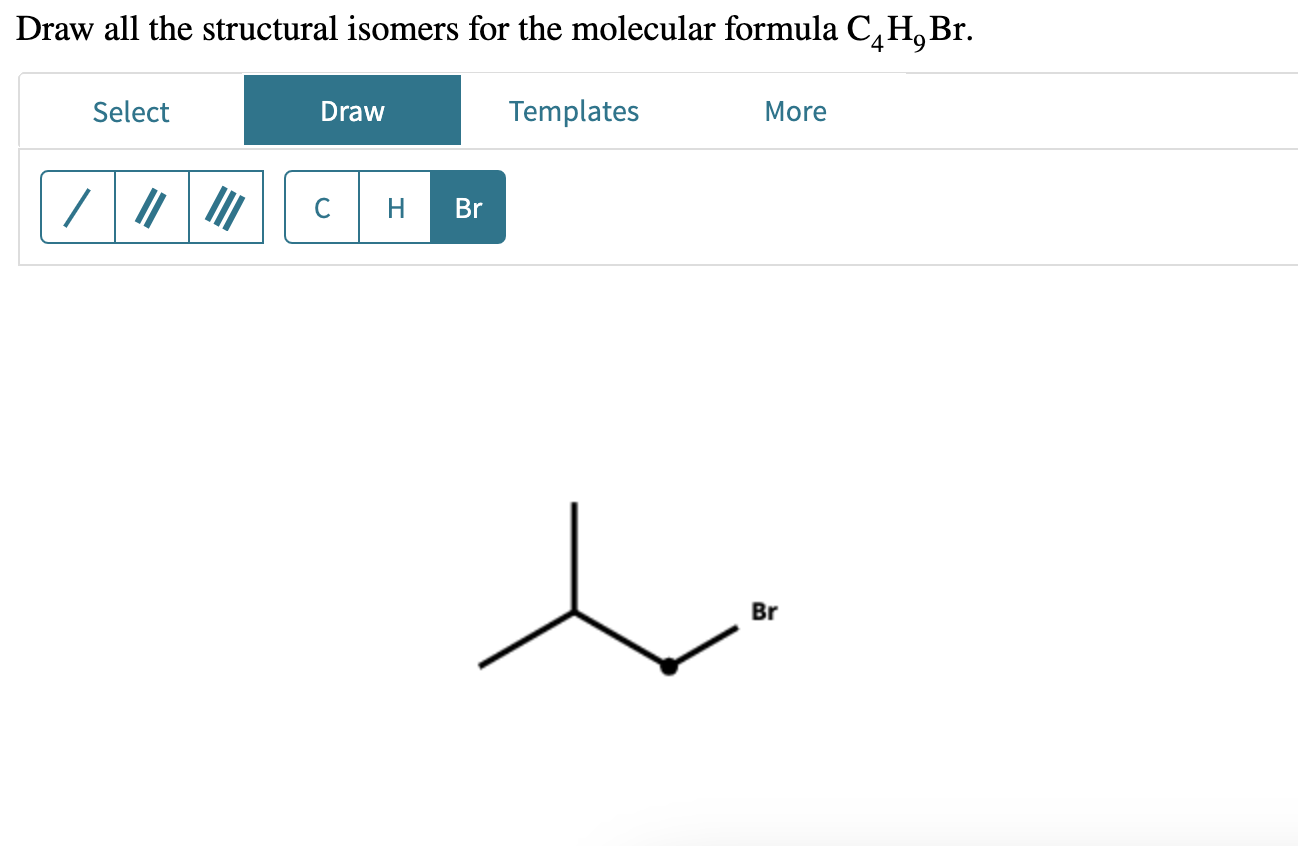
Draw All Constitutional Isomers With The Molecular Formula C4h9br

Imagine you’re at a molecular potluck, and the star dish for the evening is the humble C4H9Br. This isn't some exotic ingredient; it's just a recipe for building blocks in the vast kitchen of chemistry. But here's the fun part: the same ingredients can be assembled in a surprising number of ways, leading to totally different creations.
Think of it like baking. You've got flour, sugar, eggs, and butter. You can make a cake, cookies, or even pancakes! They all use the same basic stuff, but the arrangement is everything. That’s exactly what we’re exploring with C4H9Br – the different ways these atoms can “hang out” together.
Our main player, the bromine atom (Br), is like a playful guest at this molecular party. It loves to join the fun with its four carbon buddies (C) and nine hydrogen pals (H). But where it decides to sit at the molecular table makes all the difference.
Meet the Family: The Four Siblings of C4H9Br
So, how many different molecular personalities can C4H9Br have? Get ready, because there are four distinct ways these atoms can arrange themselves. Each one is a unique isomer, a chemical cousin that looks different but shares the same recipe.
It's like having a family with four siblings. They all have the same parents (the formula C4H9Br), but they each have their own quirks and looks. Some are tall and lanky, others are more compact, and some might have a slightly different hairstyle!
First Up: The Straight Arrow – n-butyl bromide
Our first isomer is the most straightforward, like the dependable older sibling. It’s called n-butyl bromide. Imagine a perfectly straight line of four carbon atoms, with the bromine atom happily attached to the very end.
It’s the simplest arrangement, no detours, no zigzags. Just a neat row of atoms. This one is often the first one chemists learn about because it’s so predictable.
Think of it as the classic, no-frills version of C4H9Br. It gets the job done without any fuss. It's the reliable friend who’s always there for you, in the most organized way possible.
Next: The First Branch – sec-butyl bromide
Now, things get a little more interesting. Our second isomer is sec-butyl bromide. This one is like the sibling who likes to do things a little differently, but not too wildly.
Instead of the bromine sitting at the very end, it decides to park itself on the second carbon atom in the chain. This creates a small bend, a little elbow in the molecule.
It's like having a chain of four people holding hands, but instead of holding the hand of the person next to them, the bromine holds the hand of the person two steps away. This slight change gives it a whole new personality and slightly different properties.
The Social Butterfly: isobutyl bromide
Our third isomer is the life of the molecular party, the social butterfly: isobutyl bromide. This one is a bit more branched, like a tiny, elegant tree.
Imagine three carbon atoms in a row, and then a fourth carbon atom branching off from the middle one. The bromine atom then attaches itself to the end of that main, three-carbon chain.
It's a neat little cluster. This arrangement gives isobutyl bromide its own unique dance moves in the chemical world. It’s not as straightforward as the first one, and not quite as centrally branched as the next.
The Quirky One: tert-butyl bromide
Finally, we meet our quirky, perhaps a bit eccentric, isomer: tert-butyl bromide. This one is the most compact and heavily branched of the bunch.
Picture a central carbon atom that’s acting like a hub. Three other carbon atoms are attached to this central one, forming a little tripod. Then, the bromine atom joins the party, attaching itself to that very central carbon.
It’s like a tiny, bristly ball! This highly branched structure makes tert-butyl bromide behave quite differently from its cousins. It’s the one who might surprise you with its unique talents.
Why So Many Ways? The Magic of Arrangement!
You might be wondering, why all the fuss about different arrangements? It’s because the shape and structure of a molecule dictate how it interacts with other molecules. Think of a key fitting into a lock. If the key’s shape is slightly off, it won’t work.
Similarly, these four isomers of C4H9Br, despite having the same ingredients, will react differently in chemical experiments. They might dissolve in different liquids, have different boiling points, or be more or less reactive.

It’s a beautiful reminder that even with the same basic components, endless possibilities can arise. It’s the subtle differences in how things are put together that create the diversity we see all around us, from the tiniest molecules to the grandest galaxies.
So, the next time you hear about a chemical formula, remember that it's not just a string of letters and numbers. It’s a blueprint for a tiny, invisible world with its own cast of characters, each with a unique personality and story to tell. And the C4H9Br family, with its four distinct members, is a perfect example of this molecular marvel!
The beauty of chemistry lies in these variations. It’s like discovering that the same set of LEGO bricks can build a spaceship, a castle, or a quirky robot. Each isomer is a testament to the creativity of the universe at its smallest scale.
These isomers are like siblings who share a common heritage but have forged their own paths. They might look similar at a glance, but their individual journeys and how they navigate the world of chemical reactions are distinct and fascinating.
So, next time you encounter C4H9Br, don't just see the letters and numbers. See the four unique personalities, the four ways atoms can dance and arrange themselves into something new and wonderful. It's a tiny glimpse into the immense, intricate, and often surprising world of molecular construction.
The story of C4H9Br isomers is a heartwarming tale of sameness and difference. It’s about how a shared origin can lead to diverse destinies, a universal theme echoed in every aspect of life.

It's not just about having the right ingredients; it's about how you mix them up!
This simple molecule shows us that even the smallest building blocks have their own complex personalities. They are the unsung heroes of the chemical world, quietly shaping the substances that make up everything we know.
The journey through the isomers of C4H9Br is like exploring a small neighborhood where each house, while built from similar materials, has a unique architecture and offers a different living experience. Each isomer is a character in a grand, unseen play of chemical interactions.
These variations are not just academic curiosities; they are the very reason why different chemicals have different uses and properties. Understanding these differences is key to unlocking new discoveries and innovations.
The simplicity of the formula belies the complexity of the possibilities. It’s a delightful reminder that even in the most basic concepts, there is room for immense creativity and surprise.
So, let's raise a metaphorical beaker to C4H9Br and its four fascinating isomers! They teach us that in the grand molecular mosaic, every atom’s position matters, and every arrangement tells a unique and wonderful story.
