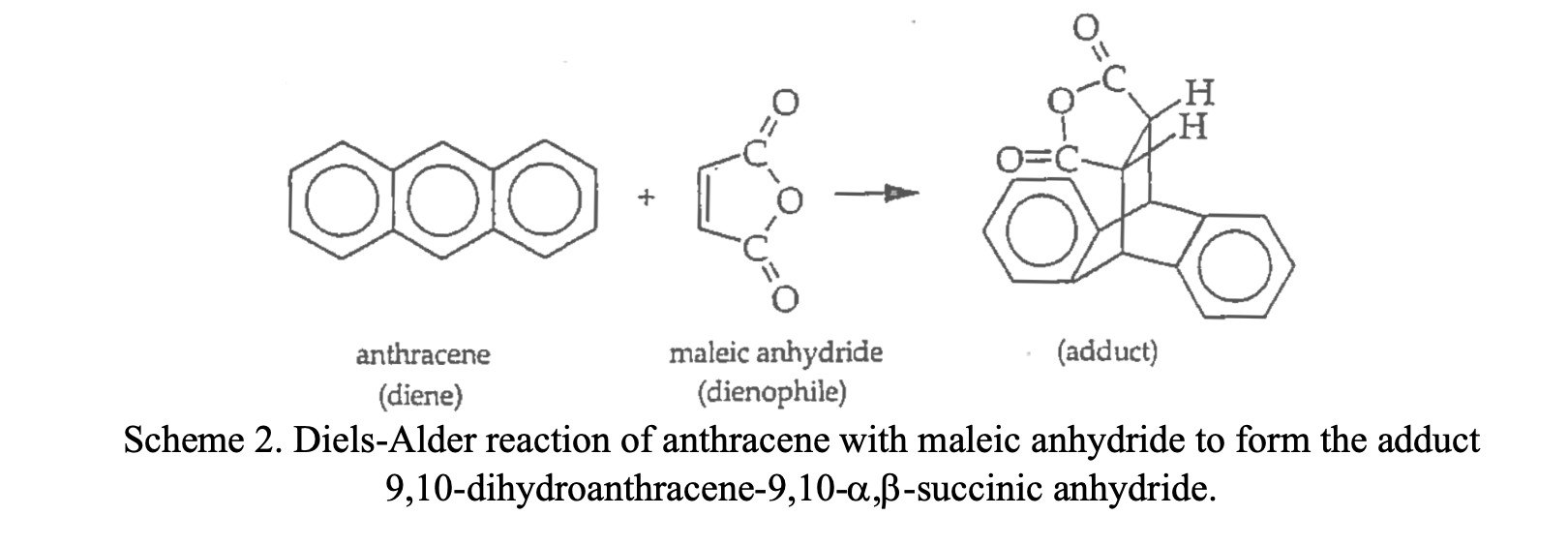
Diels Alder Reaction Of Anthracene With Maleic Anhydride Mechanism

Hey there, fellow curious minds and kitchen chemists! Ever gaze at a beautiful molecule and wonder how it all comes together? Today, we're diving into a chemical love story, a dance of molecules that’s as elegant as it is powerful. We're talking about the Diels-Alder reaction, specifically when the superstar, anthracene, meets the eager partner, maleic anhydride. Think of it as a perfectly matched pair, ready to create something brand new and exciting. No need to wear a lab coat for this one, we’re keeping it breezy, like a perfect summer afternoon.
So, what’s the big deal with the Diels-Alder reaction? It’s a way to build rings, specifically six-membered rings, by having a diene (something with two double bonds in a specific arrangement) and a dienophile (something with a double or triple bond) join forces. It’s like Lego bricks for chemists, snapping together in a predictable and beautiful way. The magic here is that it’s a concerted reaction. This fancy term just means that all the electron-shuffling and bond-forming happens in one single step. No messy intermediaries, no lingering doubts. Just pure, efficient chemical harmony.
Anthracene, our star diene, is a pretty cool molecule. It's a polycyclic aromatic hydrocarbon, meaning it's made of fused benzene rings. Think of it as a miniature, flat cityscape of carbon and hydrogen. It has a special conjugated system, which is key to its role as a diene. Its structure is like an open invitation for a partner, and maleic anhydride is more than happy to RSVP.
Maleic anhydride, on the other hand, is a cyclic anhydride. It's a bit more reactive and has a double bond that's just begging to be involved in some molecular fun. The double bond in maleic anhydride is particularly well-placed and activated, making it an excellent dienophile. It's like the perfect dance partner, ready to lead.
The Grand Unveiling: How It All Goes Down
Alright, let’s get to the heart of the matter: the mechanism. Imagine our two molecules, anthracene and maleic anhydride, swirling around. When they get close enough and the conditions are right (a little heat usually does the trick, much like getting comfortable before a good chat), they start to get serious.
The reaction kicks off with a simultaneous rearrangement of electrons. It’s a bit like a choreographed ballet. Two pi electrons from one of anthracene’s double bonds move towards maleic anhydride. At the same time, two pi electrons from maleic anhydride’s double bond move to form a new bond with anthracene. And, crucially, two more pi electrons from another of anthracene’s double bonds move to form the final bond, completing the new six-membered ring.
Think of it as a "pericyclic" reaction, meaning it happens in a cyclic transition state. All the atoms involved are essentially arranged in a circle during this key moment. It’s a bit like a group hug, where everyone contributes and everyone benefits. This concerted nature is what makes the Diels-Alder reaction so incredibly efficient and predictable.
The result? A new, fused ring system is formed. In this specific case, we get a molecule called anthracene adduct of maleic anhydride. It’s a beautiful fusion, a testament to the power of collaboration in the molecular world. The aromaticity of anthracene is slightly disrupted in the process, as one of its benzene rings transforms into a cyclohexene ring, but the overall structure is stable and fascinating.
Why Is This Such a Big Deal? (Besides Being Super Cool)
The Diels-Alder reaction is a cornerstone of organic chemistry. It's not just an academic curiosity; it's a workhorse for synthesizing complex molecules. Think of it as a foundational building block for creating all sorts of things, from pharmaceuticals to advanced materials. Many important drugs and natural products have been synthesized using this reaction, or variations of it. It's a testament to its versatility and reliability.
One of the reasons it's so popular is its stereospecificity. This means that the stereochemistry (the 3D arrangement of atoms) of the starting materials directly dictates the stereochemistry of the product. If you have a specific spatial arrangement in your diene and dienophile, you’ll get a predictable spatial arrangement in your new ring. It’s like having a precise blueprint that ensures the final construction is exactly as intended.
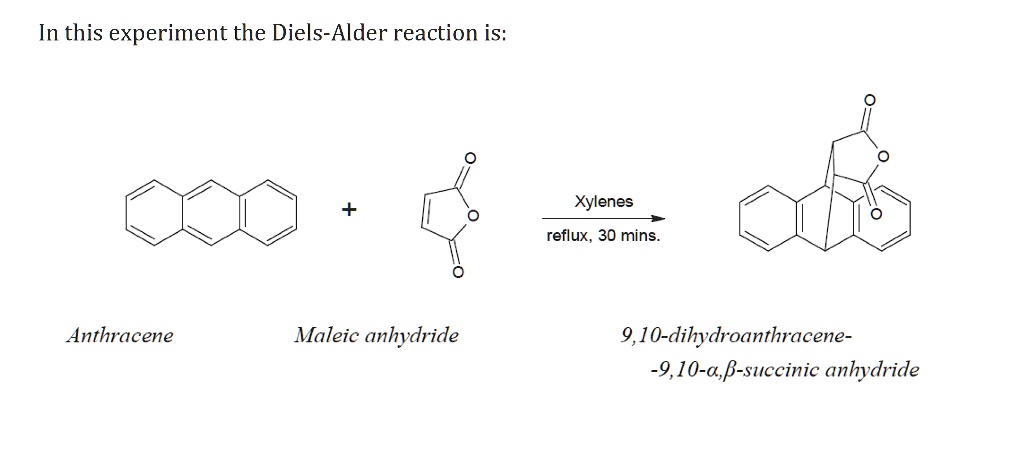
Furthermore, the reaction is often quite forgiving and can be carried out under relatively mild conditions. You don't necessarily need extreme temperatures or pressures, which is a huge plus for chemists trying to keep things efficient and safe. It’s the kind of reaction that makes you feel like a molecular artist, not a mad scientist.
Fun Facts and Practical Tips for the Everyday Chemist
Did you know? The Diels-Alder reaction was discovered by Otto Diels and Kurt Alder, who were awarded the Nobel Prize in Chemistry in 1950 for their work. They were seriously onto something! It’s always inspiring to think about those moments of discovery that fundamentally change how we understand the world.
Think about it like cooking: You wouldn't throw random ingredients into a pot and expect a gourmet meal, right? Similarly, chemists choose their dienes and dienophiles carefully. Anthracene is chosen for its specific diene structure, and maleic anhydride for its activated dienophile properties. It’s about understanding the ingredients and how they’ll interact.
For the aspiring kitchen scientist: While we don't recommend trying to synthesize complex molecules at home without proper training and equipment (safety first, always!), you can appreciate the elegance of these reactions. Think of it like appreciating a complex recipe. You can understand the steps and the purpose of each ingredient, even if you're not the one wielding the whisk.
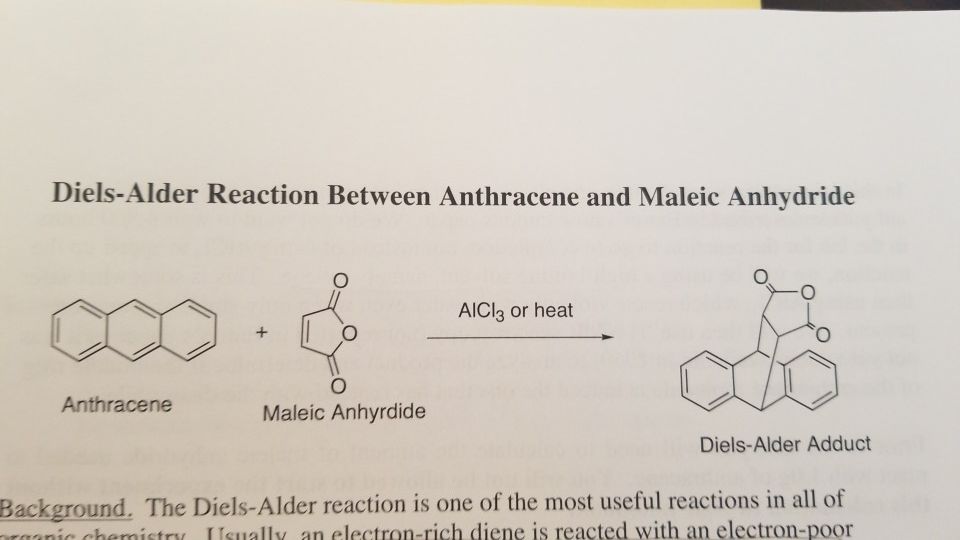
Cultural Connection: The Diels-Alder reaction is a perfect example of how science can be viewed through an artistic lens. The formation of rings, the precise dance of electrons – it’s all about structure, form, and transformation. It’s not too dissimilar from how an architect designs a building or a musician composes a symphony. There’s a deep satisfaction in understanding how complex structures are built from simpler components.
Cultural Connection 2.0: You can see echoes of this in art and design. Think about the beauty of geometric patterns in Islamic art or the precise construction of a geodesic dome. These all rely on an understanding of how basic shapes and elements can combine to create something larger and more intricate. The Diels-Alder reaction is nature’s way of doing the same with molecules.
Temperature Matters: Often, heating the reaction mixture will speed things up. This is a general principle in chemistry – more energy often means more action! But it’s a delicate balance. Too much heat can sometimes lead to unwanted side reactions or the decomposition of your molecules. It’s like finding the perfect temperature for your sourdough starter – too hot, and it dies; too cold, and it’s sluggish.
Solvent Selection: The choice of solvent can also play a role. Solvents can help dissolve the reactants and can sometimes influence the reaction rate or selectivity. It’s like choosing the right liquid medium for your artwork – water for watercolors, oil for oil paints. Each has its properties and impacts the final result.
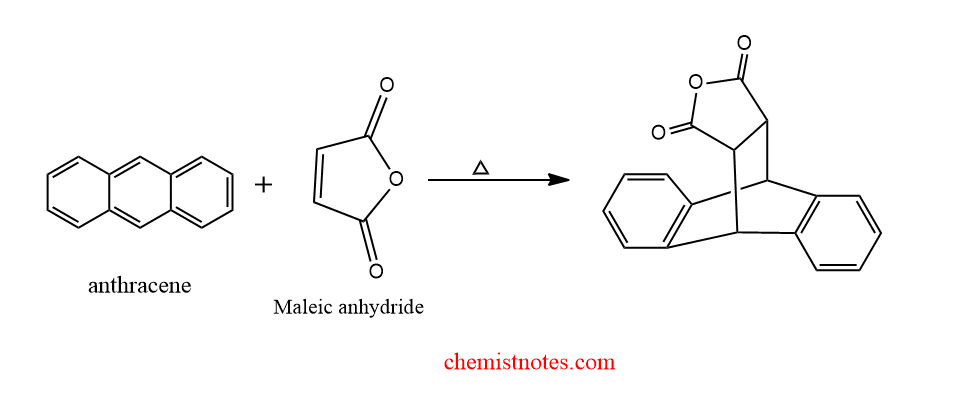
Visualizing the Magic: If you ever get a chance to see a molecular model or a 3D representation of the Diels-Alder reaction, take it! Seeing how those electron clouds interact and bonds form is incredibly illuminating. It transforms the abstract into something tangible, making the chemistry come alive. Think of it like watching a great documentary about nature – it makes you appreciate the intricate workings of the world around us.
A Moment of Reflection
Looking at the Diels-Alder reaction of anthracene and maleic anhydride, we see a beautiful illustration of how simple components can come together to form something more complex and significant. In our own lives, we're constantly engaging in our own versions of "Diels-Alder reactions." We meet new people, form friendships, collaborate on projects, and build families. Each interaction, each shared experience, is like a chemical bond being formed, creating new connections and shaping who we are.
Sometimes, these connections are instantaneous and powerful, like a well-timed Diels-Alder. Other times, they develop gradually, requiring patience and the right conditions. And just as chemists carefully select their reactants for predictable outcomes, we, too, can be mindful of the people and experiences we choose to bring into our lives. By understanding the principles of interaction and formation, we can build stronger, more fulfilling connections, creating a life that’s as elegantly constructed and exciting as a perfectly executed chemical reaction.
So, the next time you marvel at the intricate beauty of a molecule or appreciate the seamless way things come together in the world around you, remember the elegant dance of anthracene and maleic anhydride. It’s a reminder that even in the smallest corners of the universe, there's a profound beauty and logic to how things are built, and that, in its own way, is pretty darn inspiring.
