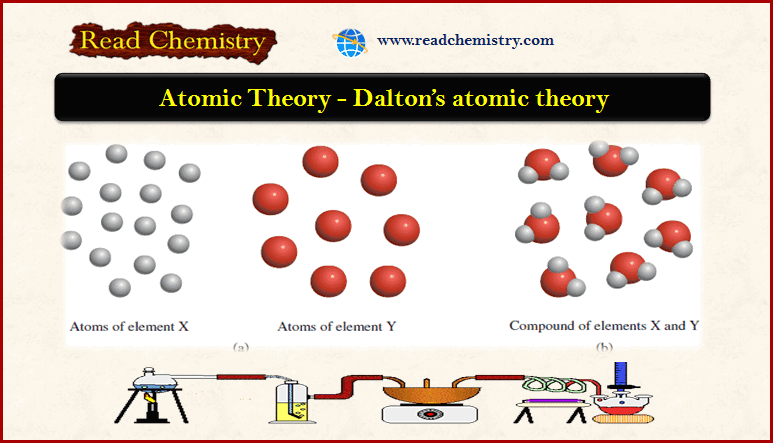
Determine Which Statements Are Consistent With Dalton's Atomic Theory.

So, I was rummaging through some old boxes in my attic the other day – you know, the usual archaeological dig of my past life. Found this dusty old chemistry textbook from high school. Remember those? Stacks of them, smelling faintly of old paper and forgotten hopes of acing that final exam. Anyway, I flipped it open, and right there, splashed across a page with a sepia-toned portrait of a rather serious-looking gentleman, was something called "Dalton's Atomic Theory."
Suddenly, I had flashbacks. Not exactly pleasant flashbacks, mind you, but definite ones. Suddenly I was back in Mr. Henderson's class, trying to wrap my adolescent brain around the idea that everything, everything, is made of tiny, indivisible particles. It felt like pure science fiction at the time, like something out of a Jules Verne novel, only with less steampunk goggles and more, well, chalk dust.
But here's the thing that struck me, staring at that textbook page with the wisdom of a few more decades (and probably a few more grey hairs) under my belt: how much of that old theory still holds up? And more importantly, how do we figure out which bits are still the "good stuff" and which bits got tossed out with the bathwater of scientific progress?
Unpacking Dalton's Ancient Wisdom (or Mostly Wisdom!)
John Dalton, our serious-looking friend, was basically the OG of modern atomic theory. He was a bit of a quiet guy, apparently. Liked observing things. And he had this radical idea: what if matter isn't just a continuous blob, but is actually made up of these super-tiny building blocks called atoms?
Think of it like this: you've got a giant Lego castle, right? You can break it down, piece by piece, until you're left with individual Lego bricks. Dalton was saying that elements, like gold or oxygen, are like those individual bricks. They're the fundamental units. Pretty neat, huh? But he went further than just saying "stuff is made of little bits." He proposed a whole set of rules, a theory, that explained how these bits behaved.
And that's where we get to the fun part: figuring out which of his pronouncements are still considered, dare I say, rock solid, and which ones have been… let's say, updated. It's like checking your old diary entries. Some things you wrote are spot on, and others make you cringe so hard you want to hide under the duvet.
Statement 1: All matter is composed of tiny, indivisible particles called atoms.
Okay, let's dive into the first of Dalton's big ideas. This one, at first glance, sounds like it's still totally true, right? We know matter is made of atoms. That's like, elementary school science 101. But then you start thinking a bit harder. "Indivisible," Dalton said. Hmm.
Here's where we gotta put on our thinking caps. While it's true that atoms are the fundamental building blocks of elements, the "indivisible" part? Yeah, that's where things get a little… fractured. We now know that atoms themselves are made up of even smaller particles: protons, neutrons, and electrons. And if you want to get really fancy, there are quarks and leptons and all sorts of subatomic shenanigans going on. So, strictly speaking, atoms aren't indivisible. They can be split, as we've seen with nuclear reactions, for example.
So, is this statement consistent? Well, it's partially consistent, but the "indivisible" bit is where it falls down. It's like saying a pizza is indivisible – technically true until someone takes a slice, right? So, when you're trying to determine consistency, you gotta look for those little loopholes, those nuances. This one, unfortunately, is a bit too flawed in its absoluteness.
Statement 2: Atoms of the same element are identical in mass and properties.
Alright, let's chew on this next one. "Atoms of the same element are identical in mass and properties." On the surface, this sounds pretty logical. If you've got two atoms of oxygen, they should be, you know, oxygen atoms. Same deal, right? Imagine a batch of perfectly identical apples from the same tree. They'd all be pretty much the same.
But science, bless its ever-curious heart, isn't always that straightforward. While atoms of the same element generally have very similar properties, they can actually have different masses. This is where the concept of isotopes comes in. Think of isotopes as slightly heavier or lighter versions of the same element. For instance, hydrogen has a common form (protium), but it also has deuterium (which has an extra neutron, making it heavier) and tritium (with two extra neutrons). They're all still hydrogen, they all behave chemically in pretty much the same way, but their masses are different.

So, this statement, like the last one, isn't entirely consistent with our modern understanding. The "identical in mass" part is the kicker. The properties? Mostly true, but the mass variation is a pretty significant detail that Dalton missed. It's like saying all twins are exactly the same – sure, they might look alike and have similar personalities, but they're still unique individuals with their own little quirks and, metaphorically speaking, weight differences.
Statement 3: Atoms of different elements have different masses and properties.
Now, this one feels like it's getting back on track. "Atoms of different elements have different masses and properties." Think about it: a gold atom is, well, a gold atom. It's going to be way heavier and behave way differently than, say, a helium atom. This makes a lot of intuitive sense, doesn't it? We don't see gold atoms suddenly deciding to act like oxygen atoms, or vice versa.
And you know what? This statement is actually pretty darn consistent with what we know today. The defining characteristic of an element is the number of protons in its nucleus, and this number directly influences its properties and, generally, its mass. While isotopes (which we just talked about!) mean atoms of the same element can have different masses, atoms of different elements are fundamentally different in their makeup and therefore their mass and how they interact chemically. This is the bedrock of the periodic table, after all!
So, when you're evaluating these statements, look for the ones that align with the fundamental distinctions between elements. This one passes the test with flying colors. It's the kind of statement that, even after all these years and all the scientific discoveries, still forms a core understanding of how the world around us is built.
Statement 4: Atoms cannot be created or destroyed in a chemical reaction. They can only be rearranged.
Here’s a biggie, and one that sounds really important for how chemistry actually works. "Atoms cannot be created or destroyed in a chemical reaction. They can only be rearranged." This is the principle of the conservation of mass, and it's a cornerstone of chemical reactions. Imagine you're baking a cake. You start with flour, sugar, eggs, and butter. You mix them up, bake them, and you end up with a cake. You haven't magically created new atoms; you've just rearranged the atoms from those ingredients into a new structure.
This statement is incredibly consistent with Dalton's atomic theory and, more importantly, with our modern understanding of chemical reactions. In any chemical change, the atoms involved are simply shuffled around to form new molecules. They don't vanish, and they don't spontaneously appear out of thin air. This is why chemical equations are balanced – to show that the number of atoms of each element on one side (the reactants) is equal to the number of atoms of that same element on the other side (the products).
It's like playing with building blocks again. You can take apart a tower and build a car, but you still have the same number of blocks. You haven't lost any, and you haven't gained any. This principle is so fundamental that it's hard to overstate its importance. So, yes, Dalton absolutely nailed this one. It's a keeper.
Statement 5: Atoms of different elements combine in simple, whole-number ratios to form compounds.
Last but not least, let's look at this final postulate. "Atoms of different elements combine in simple, whole-number ratios to form compounds." What does that even mean? Well, think about water. We know water is H₂O. That's two hydrogen atoms for every one oxygen atom. That's a 2:1 ratio, a nice, simple, whole number. Or carbon dioxide, CO₂ – one carbon atom to two oxygen atoms (a 1:2 ratio).
Dalton observed this pattern and proposed that compounds are formed when atoms of different elements join together in fixed, whole-number proportions. This is the law of definite proportions and the law of multiple proportions in action. It’s why we have specific chemical formulas for compounds and not, say, a random jumble of atoms.
And guess what? This statement is also wonderfully consistent with our current chemical knowledge. The way atoms bond together follows very specific rules, and these rules typically result in these neat, whole-number ratios. Of course, there are some very complex molecules and some edge cases in advanced chemistry, but for the vast majority of compounds we encounter, this principle holds true. It's like saying that when you build with Lego, you usually end up with recognizable structures, not just random piles of bricks. Dalton's insight here was crucial for understanding how elements combine to create the vast diversity of substances we see in the world.
So, What's the Verdict?
When you're faced with these kinds of questions, remember to approach them like a detective. You've got the clues (Dalton's statements), and you've got the modern evidence (your current understanding of chemistry). Some statements will be direct hits, others will be close but not quite, and some might be way off the mark.
Dalton's theory was revolutionary for its time, and much of it still forms the bedrock of our understanding of matter. However, science is a journey of constant refinement. We build upon the foundations laid by pioneers like Dalton, and as our tools and knowledge expand, we gain a more nuanced and accurate picture of the universe. So, the next time you’re staring at a textbook, or even just looking at a lump of… well, anything, take a moment to appreciate how far we've come in understanding the tiny, fascinating world of atoms!
