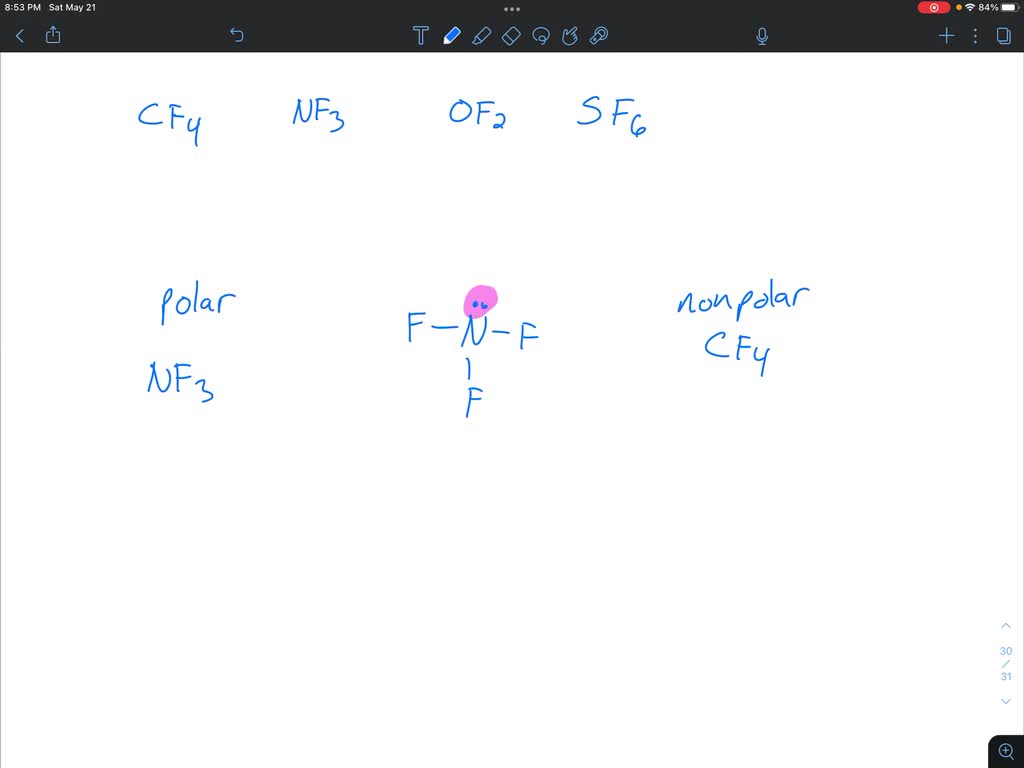
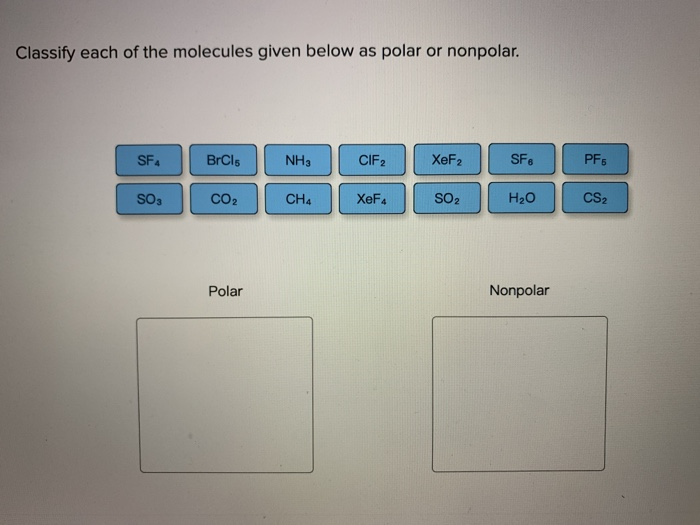
Determine Whether Each Molecule Given Below Is Polar Or Nonpolar.
Alright, gather 'round, you science curious cats and kittens! We're about to embark on a thrilling, albeit slightly nerdy, adventure into the wacky world of molecular polarity. Forget those stuffy lab coats and intimidating textbooks, because we're doing this the good old-fashioned café gossip way. Imagine me, sipping a perfectly frothy cappuccino, pointing at a sugar cube and saying, "Now, that little guy, is he a party animal or a lone wolf?" That's the vibe we're going for.
So, what in the electronegative heck is "molecular polarity" anyway? Think of molecules as tiny little teams of atoms, all holding hands. Now, some atoms are like the super popular kid in school, always hogging the attention (and the electrons). These are your electronegative atoms. Others are a bit more chill, content to share. This electron-sharing business is the crucial part. If the electron-sharing is like a fair and balanced potluck dinner, everyone gets a good bite. But if one atom is a greedy guts, snatching all the electron-goodies, things get a little… lopsided.
This lopsidedness is what we call polarity. Imagine a tiny tug-of-war happening within the molecule. If one side is winning (electronegativity difference!), you've got a polar molecule. It's got a positive end and a negative end, like a tiny, invisible battery. If the tug-of-war is a draw, a perfectly symmetrical stalemate, then you've got a nonpolar molecule. They're the Switzerland of the molecular world – neutral and unbothered.
Now, how do we figure this out without actually getting our hands dirty with electron microscopes? We use a little bit of brainpower and a dash of visual intuition. We look at the molecule's shape and the types of atoms involved. It's like trying to guess if a couple is going to have drama based on their body language – you can tell a lot from how they're positioned!
Let's start with some absolute legends of the molecular universe. First up, the undisputed champion of everyday life: Water (H₂O). Oh, water. The stuff of life, the destroyer of empires, and the key ingredient in my morning Earl Grey. Is it polar or nonpolar? Drumroll, please! It's a resounding POLAR! And here's why: Oxygen is a real electron-hoarder. It's like the Beyonce of electronegativity, demanding all the attention. Hydrogen, bless its little heart, is more like a willing fan, happy to share its electrons. Because of this unequal sharing, the oxygen end of the water molecule gets a slightly negative charge, and the hydrogen ends get a slightly positive charge. It's got that bent-over shape, too, which means the charges don't cancel out. It's a molecule with a distinct personality, a little bit of a diva, if you will.

This polarity is why water is such an amazing solvent. It can dissolve all sorts of other polar things, like salt and sugar. Think of it as a magnet for other charged particles. Nonpolar things? Water basically rolls its eyes and says, "Nah, not my type." That's why oil and water don't mix – oil is nonpolar, and water is like, "Get away from me, you bland individual!"
Now, let's pivot to a molecule that's the polar opposite (pun intended!) of water's dramatic flair. Meet Methane (CH₄). This is your quintessential NONPOLAR molecule. Carbon here is pretty chill about electron sharing, and the four hydrogens are equally chill. Crucially, methane has a perfectly symmetrical, tetrahedral shape. It's like a perfectly balanced seesaw. Even though there are slight differences in electronegativity between carbon and hydrogen, the symmetrical arrangement means all those tiny pulls cancel each other out. It's the molecular equivalent of "no hard feelings." Methane is happy to float around, minding its own business. It's the understated friend who's always there, never causing a fuss. Fun fact: Methane is a major component of natural gas and also what cows burp out. So, you could say it's also involved in… digestive affairs.
Let's try another one. How about Carbon Dioxide (CO₂)? This one's a bit of a trickster. On the surface, you might think, "Oxygen is electronegative, carbon is in the middle, this must be polar!" But wait, there's a twist! CO₂ is a linear molecule. It's like a perfectly straight line of atoms: O-C-O. The oxygen atoms are pulling electrons equally in opposite directions. It's another one of those perfect tug-of-war stalemates. So, despite the electronegativity difference, the symmetry of the molecule means all the charges cancel out. Therefore, CO₂ is NONPOLAR. It’s the chameleon of molecules, looking like it should be polar, but in reality, it’s a master of disguise, achieving perfect neutrality. This is why dry ice (solid CO₂) doesn't really dissolve in water in the same way polar substances do.
Moving on to Ammonia (NH₃). This one’s got a bit of a nitrogen-induced kick. Nitrogen is significantly more electronegative than hydrogen. It's like the nitrogen is the captain of the ship, and the hydrogens are the loyal crew members, handing over all the navigational charts (electrons). Ammonia has a trigonal pyramidal shape, kind of like a little tripod with a lone pair of electrons sitting on top. This shape, combined with nitrogen's greediness, means there's a clear separation of charge. The nitrogen end is negative, and the hydrogen ends are positive. You guessed it: Ammonia is POLAR! It's another one that's great at dissolving things, especially things that are also polar.

Let's spice things up with Ethanol (C₂H₅OH). This is the alcohol in your favorite beverage (responsibly, of course!). It's a bit of a hybrid situation. You've got the nonpolar carbon chain (the "ethyl" part), which likes to hang out with other nonpolar things. But then you have the hydroxyl group (-OH), which is very much like water – the oxygen is electronegative, making that part of the molecule POLAR. So, is ethanol polar or nonpolar? It's kinda both, but we usually classify it as POLAR because of the significant polarity of the -OH group. This duality is why alcohol can dissolve both polar and nonpolar substances to some extent. It's the ultimate molecular social butterfly!
Finally, let's consider Benzene (C₆H₆). This is a ring of six carbon atoms, each bonded to one hydrogen. Benzene is a classic example of a NONPOLAR molecule. The ring structure is incredibly symmetrical, and while there are slight differences in electronegativity, the perfect hexagonal arrangement means everything cancels out. It's like a perfectly choreographed dance where everyone ends up back in their original spot. Benzene is a fundamental building block in many organic compounds and is known for its stability. It’s the stoic rock of the organic world, unfazed by electron fluctuations.
So, there you have it! The next time you're wondering about a molecule's personality, just remember: look at the atoms involved, their electronegativity differences, and the molecule's shape. Is there a tug-of-war? Is it symmetrical? Is it a diva or a peacekeeper? With a little practice, you'll be a molecular polarity pro, able to identify these tiny personalities faster than you can say "electronegativity." Now, who wants another coffee?
