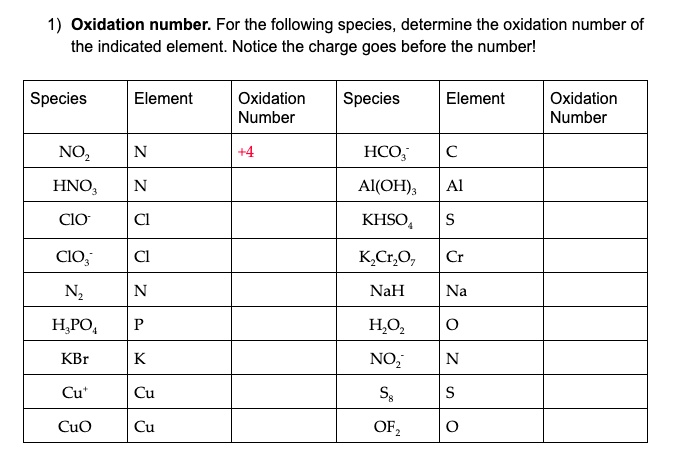
Determine The Oxidation State Of Each Of The Following Species.

Let's be honest. When someone mentions "oxidation states," your brain probably conjures images of dusty textbooks and complicated formulas. Mine too. It's like that friend who insists on explaining the plot of a movie you've already seen, but with more numbers. But what if I told you we could actually have a little fun with this? A tiny bit of fun, mind you. We're not talking rollercoasters or skydiving, but more like finding a perfectly ripe avocado. Small victories, people.
Think of oxidation states as the "jobs" assigned to atoms in a chemical compound. Some atoms are natural-born leaders, always wanting to be in charge. Others are happy to follow along. And some are just trying to get by, doing their best with what they've got. It's basically a chemical soap opera, and we're here to be the gossip column.
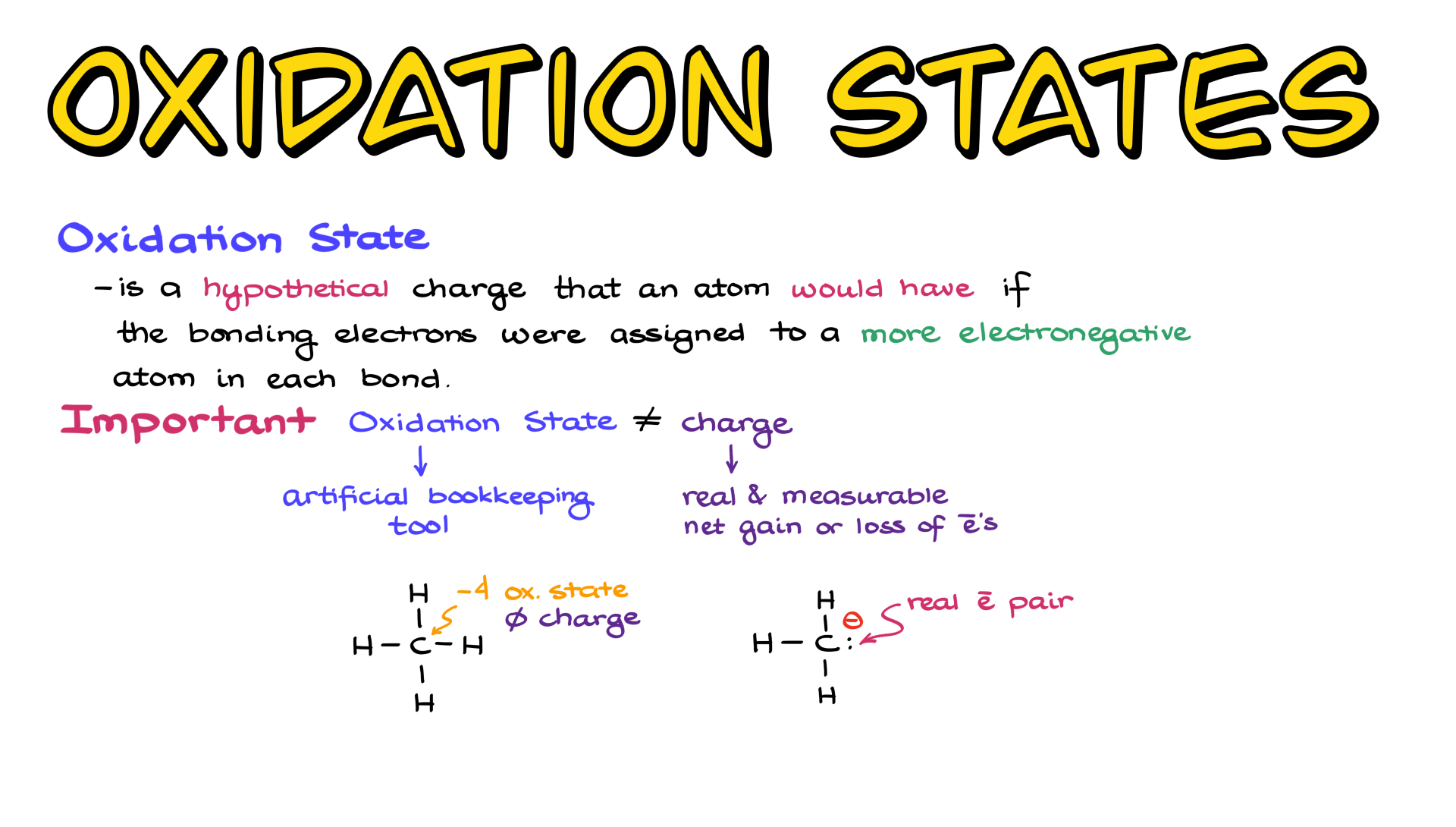
So, let's dive into this electrifying world. Our first contender for "most likely to be misunderstood" is water, or H₂O. We see it everywhere, right? But even this humble molecule has its secrets. You've got your hydrogen atoms. They're usually pretty chill. Most of the time, they're happy with a +1 job. It's like a participation trophy in the chemical world. They show up, they do their thing. Easy peasy.
Then there's the big boss: oxygen. Oxygen is a bit of a drama queen. It loves to be in charge. So, it usually rocks the -2 job. It's like the queen bee of the hive, always pulling the strings. In water, our two hydrogen buddies are busy with their +1s, and our oxygen queen is doing her -2 thing. Add them all up, and what do you get? Zero. A perfectly balanced chemical kingdom. See? Not so scary.
Now, let's crank up the heat a little. What about sodium chloride? That's just fancy talk for table salt, NaCl. This one is like that classic buddy-cop movie. You've got sodium, which is a big softie. It's always eager to give away an electron, so it gets a +1 job. It's like the friendly neighbor who always lends you a cup of sugar. And then you have chlorine. Chlorine is a bit more intense. It's always looking to grab an electron, hence its -1 job. Together, they make the ultimate dynamic duo, forming the salt we love to sprinkle on everything. A perfect +1 and -1. Boom. Done.
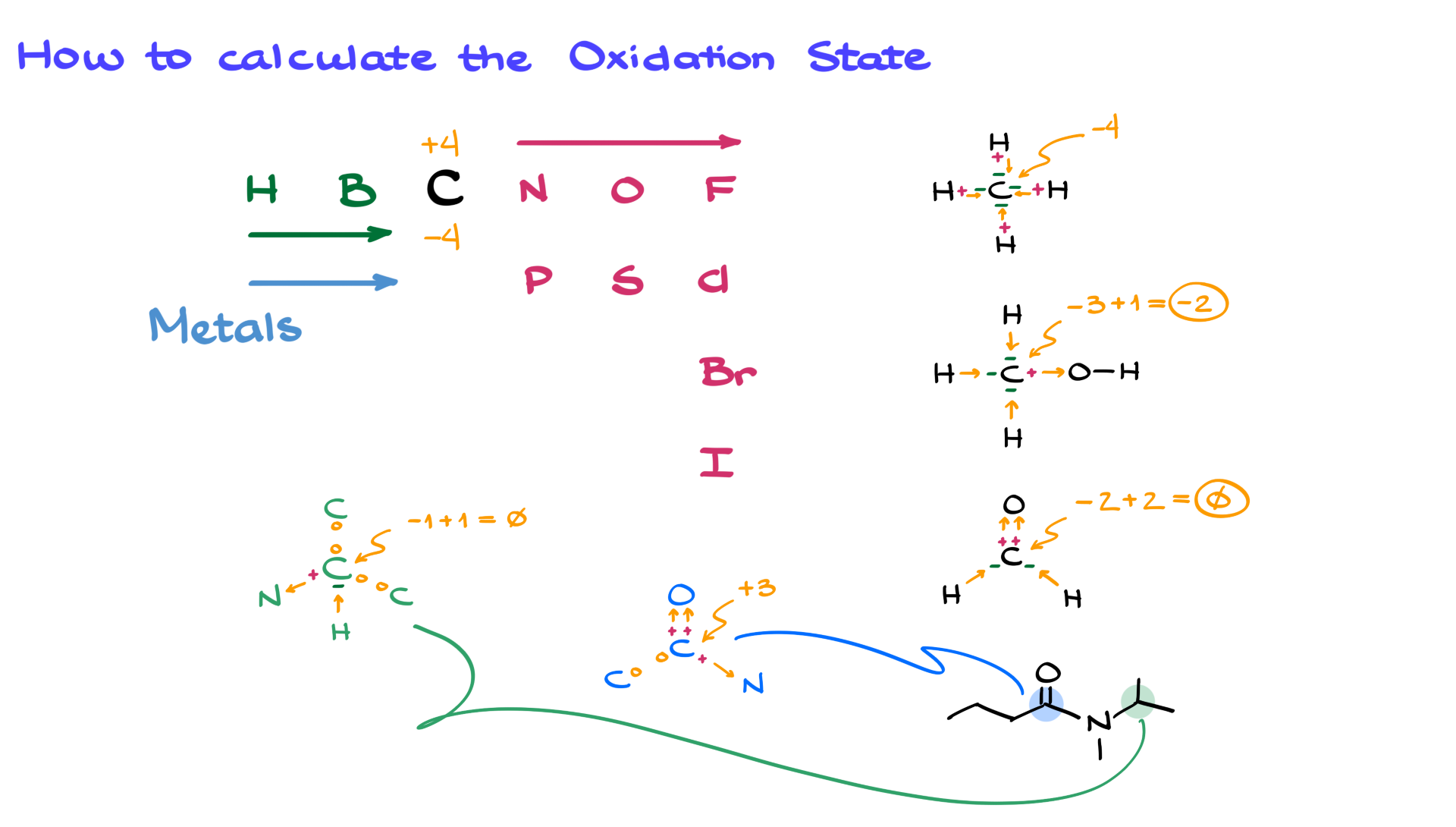
Let's try something a little more adventurous. How about the permanganate ion, MnO₄⁻? This one sounds like it belongs in a sci-fi movie, doesn't it? We've got our familiar friend, oxygen, doing its usual -2 gig. There are four of them, so that's 4 times -2, which equals -8. Now, the whole thing has a charge of -1. This means that our manganese atom has to pick up the slack. It's like the one responsible friend in a group of four. To get from -8 to -1, manganese needs to be a +7. It's carrying a lot of weight, that manganese! But it gets the job done.
Then we have something like sulfuric acid, H₂SO₄. We've got our two hydrogens, each happily doing their +1 thing, so that's +2. We also have our four oxygens, each doing their -2 thing, totaling -8. Now, the whole molecule is neutral, meaning it has a total charge of 0. So, we have +2 from the hydrogens and -8 from the oxygens, which adds up to -6. To make the whole thing zero, our sulfur atom has to be a +6. It's the mediator, the peacemaker of the molecule. It has to balance out all the other atoms' demands.
It's almost like assigning roles in a play. Hydrogen is often the cheerful supporting actor, happy with a +1. Oxygen is the leading lady, usually demanding a -2. And the other elements? They're the versatile cast members, taking on whatever role is necessary to make the play (or chemical compound) work. Sometimes they're the heroes, sometimes the villains, and sometimes they're just trying to remember their lines.
And you know what? It's okay if it feels a little bit like guesswork sometimes. Because at its heart, determining oxidation states is just about finding that perfect balance. It's about making sure all the numbers add up. It's the chemical equivalent of making sure your socks match before you leave the house. A small but important detail.
Oxidation States in Organic Molecules — Organic Chemistry Tutor
Let's take a look at the sulfate ion, SO₄²⁻. Again, oxygen is doing its -2 job, four times making -8. The whole ion has a charge of -2. So, the sulfur here needs to be a +6 to bring the total charge to -2 (-8 + 6 = -2). It's like the calculator of the group, making sure everything adds up. It’s not always the flashiest role, but it’s essential.
What about the nitrate ion, NO₃⁻? We have our three oxygen atoms, each at -2, for a total of -6. The overall charge of the ion is -1. So, our nitrogen atom has to be a +5. It’s the one making the tough decisions to keep the group in line. A +5 is a pretty high oxidation state. This nitrogen is clearly a go-getter.
It's like a chemical puzzle, and each atom has its assigned spot. You've got the predictable players, like our trusty hydrogen and oxygen. Then you have the more eccentric characters, the ones who can take on a variety of roles. And the goal? To make sure the whole thing is electrically balanced. It's the unsung hero of chemistry, this whole oxidation state business. It might not be as glamorous as inventing a new element, but it’s the glue that holds so many chemical reactions together. So next time you see a chemical formula, don't just see a jumble of letters and numbers. See a little chemical drama unfolding. And maybe, just maybe, crack a smile.