Determine The Hybridization State Of Each Carbon Atom In Nemotin.

Alright folks, gather 'round, grab your lattes, and try not to spill your biscotti because we're about to dive headfirst into the wonderfully weird world of Nemotin! Now, Nemotin, if you haven't heard, is this thing that’s apparently a super cool natural compound. Think of it as nature's own little molecular ninja, supposedly doing all sorts of neat tricks. But today, we're not here to talk about its celebrity gossip or its workout routine. Nope, we're here to get down and dirty with its carbon atoms and figure out their hybridization state. Sounds fancy, right? It's basically asking each carbon atom: "Hey buddy, what kind of party are you throwing with your electrons?"
Now, before you start picturing tiny carbon atoms in little party hats, let's get real. We're talking about chemistry. And chemistry, as you know, can sometimes feel like trying to assemble IKEA furniture with instructions written in ancient hieroglyphics. But fear not! We're going to make this as fun as discovering a forgotten bag of chips in your car's glove compartment. Because frankly, who doesn't love a good surprise? And Nemotin, my friends, is full of surprises.
The Grand Carbon Census: Meet the Usual Suspects
So, why are we even bothering with these hybridization states? Well, each carbon atom in Nemotin has a specific "personality" based on how it’s bonded to other atoms. This personality dictates how it behaves, how it interacts, and basically, its entire social calendar in the molecular world. It’s like knowing if your friend is a chill homebody (sp3) or a wild rave-goer (sp) – it tells you a lot about what to expect!
There are three main players in the hybridization game for carbon: sp3, sp2, and sp. Think of them as different types of dance moves. The sp3 is your classic, reliable shuffle – nice and steady, making four single bonds. The sp2 is a bit more flamboyant, doing a fun little side-step that involves a double bond and two single bonds. And the sp? That’s the daredevil, the one doing the splits with a triple bond and one single bond, or two double bonds. High-energy stuff!
The sp3 Shuffle: The Backbone of Nemotin's Chill Vibes
Let’s start with the most common guy, the sp3 hybridized carbon. These are your everyday, no-fuss carbon atoms. They’re like the comfy sweatpants of the molecular wardrobe. They’ve got four single bonds going on, and they’re super happy just chilling out, forming the backbone of pretty much everything. In Nemotin, you’ll find a bunch of these guys holding hands (or rather, sharing electrons) with hydrogen atoms or other carbon atoms in single bonds.
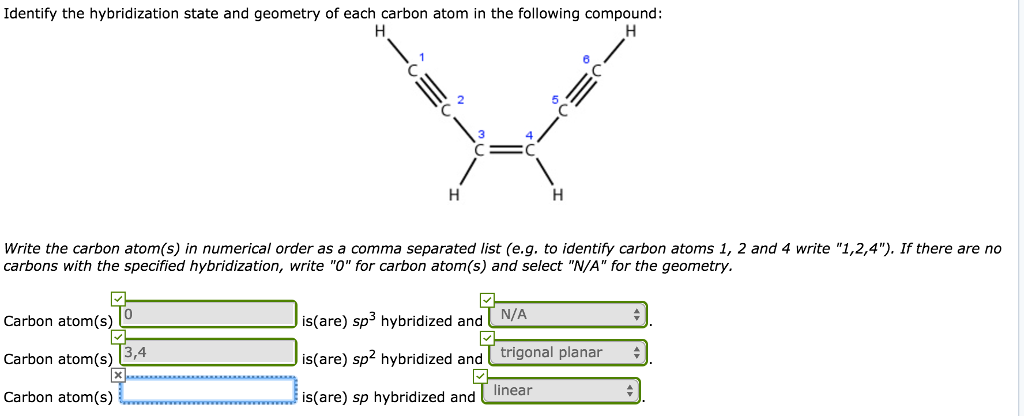
Imagine a long chain of these sp3 carbons, like a ridiculously long baguette. Each one is perfectly content, offering a stable foundation. They’re the reliable friends who always show up on time. They're the ones making sure Nemotin doesn’t just spontaneously combust. They’re the unsung heroes, really. Without them, Nemotin would be… well, it wouldn’t be Nemotin. It might just be a cloud of excited, disorganized carbon dust, which frankly, sounds like a terrible party.
Here’s a fun fact for ya: The sp3 hybridization results in a tetrahedral geometry. That means the atoms around it are arranged in a shape like a pyramid with a triangle base. Think of a fancy crystal, but way tinier and a whole lot less sparkly. It’s all about spreading out those electron clouds to get maximum personal space. Even molecules want their own bubble, you know?
The sp2 Side-Step: Where the Action Gets a Little More Interesting
Now, things get a bit more jazzy when we meet the sp2 hybridized carbons. These are the ones who decided to spice things up. Instead of four single bonds, they’re rocking one double bond and two single bonds. That double bond? It’s like a secret handshake, a more intense connection. These guys are often found in rings or structures where there’s a bit of extra ‘oomph’ needed.
Think of the sp2 carbons as the ones who brought the disco ball to the party. They’re responsible for those double bonds, which are crucial for a lot of chemical reactions. They’re not as rigid as the sp3 carbons; they have a little more wiggle room, a bit more flexibility. This is where Nemotin might start flexing its chemical muscles, if it had any.
These sp2 carbons are typically found in structures that are planar. That means they lie flat, like a pancake. They’re arranged in a triangle around the carbon atom. Imagine a perfectly formed slice of pizza, with the carbon at the center. It’s a neat and tidy arrangement, but with that extra double bond for flair. These are the carbons that can get into all sorts of exciting chemical dances, like adding new partners or swapping them out. They’re the social butterflies of the carbon world!
The sp Daredevils: The High-Wire Acts of Nemotin
And then, we have the absolute thrill-seekers: the sp hybridized carbons. These are the daredevils, the ones who are practically walking a tightrope. They’re involved in either a triple bond and one single bond, or two double bonds. Talk about living on the edge! These guys are less common in many organic molecules, but when they show up, you know things are about to get interesting.
In Nemotin, if you spot an sp hybridized carbon, it’s like finding a unicorn doing a handstand. These carbons are responsible for forming linear structures. The bonds shoot out in opposite directions, like two arrows fired from a bow. They’re the straight-line thinkers, the ones who get right to the point. They have maximum overlap with their neighbors, which makes them super strong and stable in their own unique way.
The geometry here is linear. Imagine a perfectly straight stick. That's the vibe. These carbons are all about efficiency and directness. They’re not about frills or fancy footwork; they’re about getting the job done with maximum impact. They’re the ninjas of the carbon world, quick, precise, and deadly efficient (in a chemical sense, of course!).
So, What's Nemotin's Hybridization Hot Mess (or Hotness)?
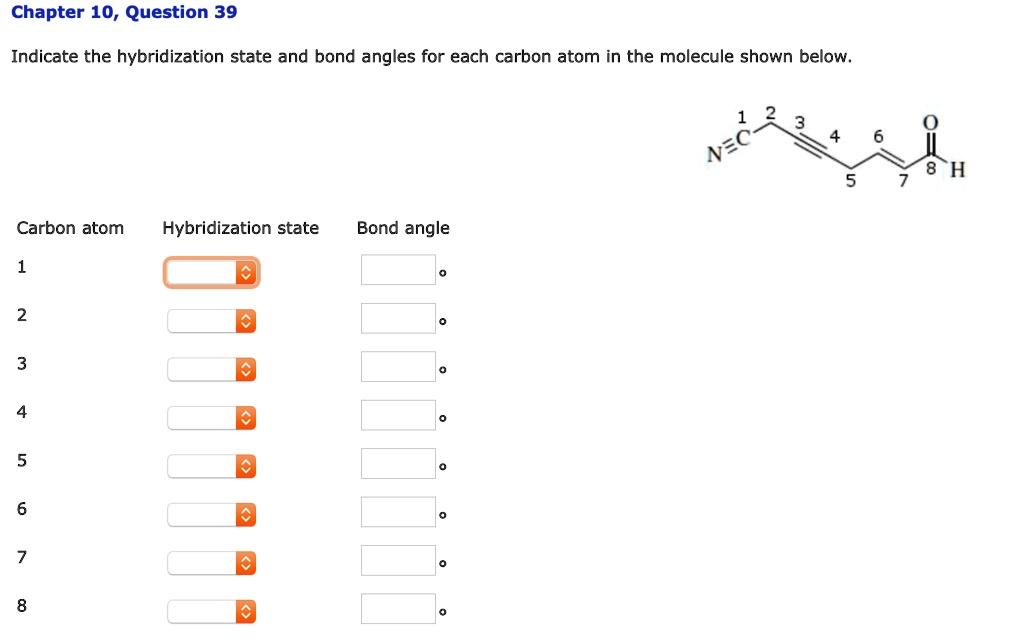
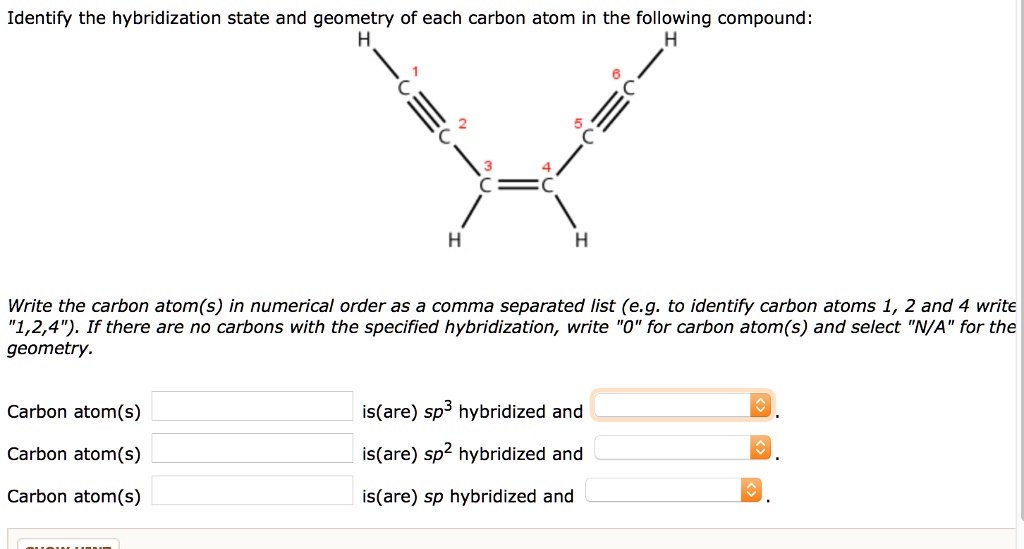
Now, to actually determine the hybridization state of each carbon atom in Nemotin, you'd need to look at its chemical structure. It’s like being a detective, but instead of clues, you're looking at lines and letters. You count the number of atoms each carbon is bonded to and the number of bonds it has.
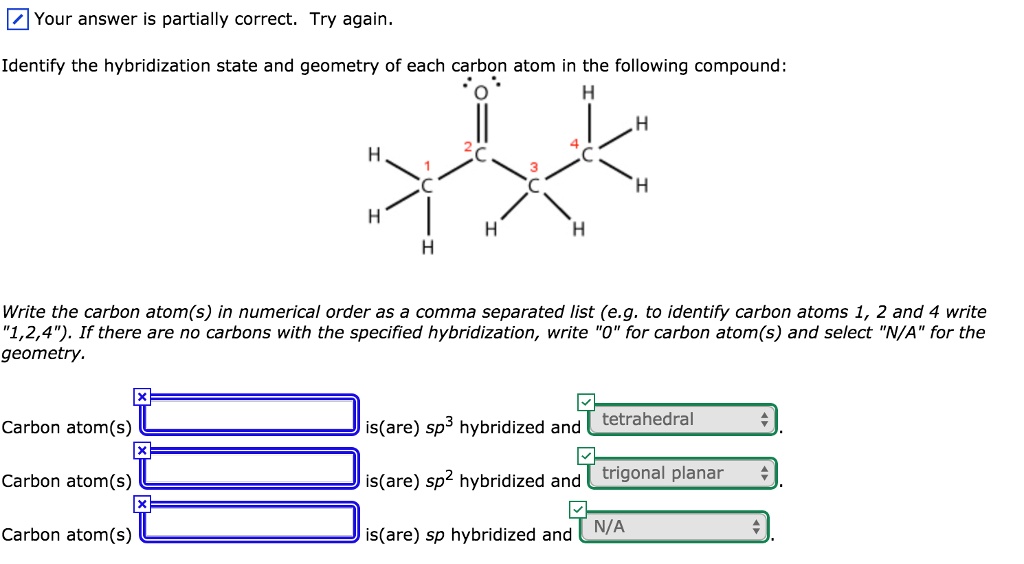
If a carbon atom has four single bonds, bam! it’s sp3. If it has one double bond and two single bonds, pow! it’s sp2. And if it has a triple bond or two double bonds, wham! it’s sp. It's like a simple math problem, but with atoms and electron clouds. Who knew algebra could be so… organic?
Nemotin, being a natural product, likely has a mix of these. It’s not going to be all sp3 like a boring alkane, nor will it be a pure sp2 fiesta like benzene. It’s probably got the stable sp3 backbone, some sp2 regions for reactivity and structure, and maybe, just maybe, a rogue sp carbon if nature decided to get particularly creative. The exact arrangement will dictate its properties, making it the unique molecule it is.
So, the next time you hear about some fancy natural compound like Nemotin, remember that behind all the impressive claims, there’s a bunch of carbon atoms doing their thing, each with its own unique hybridization state. It’s a little world of electron sharing and bonding, and frankly, it’s way more fascinating than trying to fold a fitted sheet. And with that, I’ll leave you to ponder the electrifying lives of carbon atoms. Cheers!
