Determine The Best Formula For Reacting Elements Calcium And Iodine

Hey there, fellow science enthusiast! Grab your favorite mug, because we're about to dive into something super cool, but in a totally laid-back way. You know, the kind of stuff that makes you go, "Huh, neat!" over your morning brew. We're talking about making a chemical reaction happen. Specifically, the one between calcium and iodine. Ever wondered what happens when these two buddies meet? It's not quite like meeting your favorite celebrity, but in the chemical world, it's pretty darn exciting!
So, calcium. What's the deal with calcium? Think of it as that sturdy, reliable friend. It's everywhere, really. In your bones, in milk (gotta have that calcium!), in chalk. It's a metal, and metals, well, they like to be a bit generous. They like to give away electrons. It’s like they’re saying, "Here, take this! I have plenty!" Calcium, in particular, is pretty keen on parting with two of its electrons. It's a real electron-sharing extrovert, this calcium.
And then there’s iodine. Iodine is a bit different. It’s a non-metal, a halogen. Halogens are the ones who are always looking for a handout… of electrons, that is. They're like the opposite of calcium. They want to grab electrons. They’re a bit more… needy, in a good chemical way. Iodine, being a halogen, is happy to snatch up one electron. Just one. It’s not as greedy as some of its halogen cousins, but it’s definitely on the electron-collecting team.
So, we have calcium, the electron giver (two of them, remember!), and iodine, the electron receiver (just one). This already smells like a party, right? When you put two things together that have such opposite desires when it comes to electrons, things are bound to get interesting. It’s like putting a magnet next to a piece of metal – there’s a natural attraction there. Chemistry often boils down to this whole give-and-take of electrons. It’s the universe’s way of keeping things balanced, I guess. Who knew the cosmos was so into equilibrium?
Now, the big question, the million-dollar question (though it’s way cheaper than that in reality): what’s the formula when these two get together? What’s the best formula? This isn't a "try this on a whim" situation, oh no. Chemistry formulas are like recipes. You gotta get the ingredients (or in this case, the elements) in the right amounts, or you end up with… well, something that’s not quite what you intended. And in chemistry, that could be anything from a fizzle to a pop, or just a sad, unreacted mess.
We need to think about how many calcium atoms and how many iodine atoms are going to be buddies. Remember our electron talk? Calcium wants to lose two electrons. Iodine wants to gain one. If we have one calcium atom and just one iodine atom, the calcium is going to be left with one extra electron it wants to get rid of. That's not a happy camper situation for the calcium. It’s like bringing a whole pizza to a party where everyone’s only hungry for a single slice. There’s just… leftover pizza.
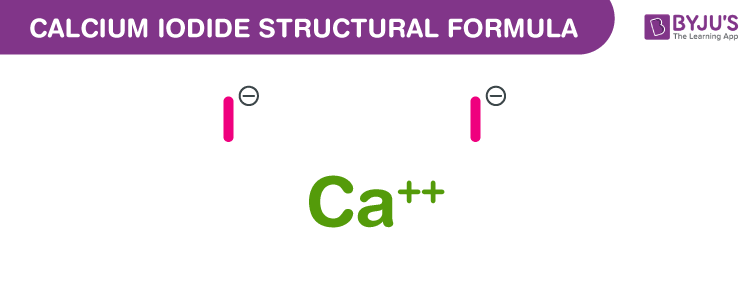
So, how do we make sure everyone’s satisfied? We need to balance those electrons! If one calcium atom is offering up two electrons, and each iodine atom can only take one, then it makes perfect sense, doesn't it, that we'd need two iodine atoms to happily scoop up those two electrons from a single calcium atom. It's a perfect match! Two little iodine friends can each grab one electron from the big-hearted calcium. Problem solved!
This is where the formula comes in, and it’s actually super elegant. We represent calcium with the symbol 'Ca'. Simple enough, right? And iodine? That's 'I'. But we need to show how many of each we have in our final compound. Since one calcium atom needs to pair up with two iodine atoms, our formula looks like this: CaI₂. See that little '₂' next to the 'I'? That’s our signal. It tells us there are two iodine atoms for every one calcium atom. It’s the chemical handshake, the chemical handshake that works!

And what do we call this glorious creation? When calcium and iodine get together in this specific ratio, they form a compound called calcium iodide. Sounds fancy, right? But it's just the name we give to this happy union. It’s not like they get married and change their last names, but in the chemical world, this is about as close as it gets to a stable, committed relationship.
Now, let's talk about why this happens. It's all about achieving stability. Elements are a bit like us; they don't always like being in a state of high energy or imbalance. They want to be comfortable, to have a full outer electron shell. For calcium, losing those two electrons gets it to that nice, stable electron configuration. For iodine, gaining one electron does the same trick. So, CaI₂ is the most stable arrangement for these two elements to exist together.
Think of it like this: if you’re building with LEGOs, and you have these specific pieces that only fit together in a certain way. You can’t just jam any old pieces together and expect a sturdy castle, can you? You need the right pieces, in the right places. CaI₂ is the perfect LEGO build for calcium and iodine.
What if we tried a different ratio? Like CaI? Well, as we discussed, the calcium would be holding onto an extra electron. It’s not a stable situation. The compound would likely break apart, or it just wouldn't form in the first place. Nature, bless its heart, is pretty efficient. It doesn’t like wasted energy or unbalanced arrangements. So, it nudges things towards the most stable form, which is CaI₂.
Or what about CaI₃? That would mean one calcium is trying to get rid of three electrons, but it only has two to give! And we'd have three iodines trying to grab a total of three electrons, but only getting two from the calcium. That's just… chaos. Utter chemical confusion. So, no, CaI₃ is not on the menu. The universe isn't that messy, thankfully.
So, to recap our little coffee chat: calcium, the generous metal that likes to donate two electrons. Iodine, the electron-snatching halogen that happily takes one. To make them happy and stable together, we need two iodines for every one calcium. This gives us the formula CaI₂, calcium iodide. It’s the chemical sweet spot, the perfect balance. It’s the formula that just works, ensuring both elements achieve their desired electron configurations and form a stable compound.

It’s really quite beautiful when you think about it. This fundamental principle of electron sharing, or in this case, electron transfer, dictating the very structure of the matter around us. From the chalk on a blackboard to the minerals in your multivitamin, these simple rules are at play. And CaI₂ is just one perfect example of this grand cosmic dance of atoms.
So, next time you're thinking about chemical reactions, remember calcium and iodine. Remember that little '₂' and what it signifies. It's not just a number; it's the key to unlocking a stable, happy compound. It’s the difference between a successful chemical marriage and a messy, unfulfilled fling. And who wants a messy chemical fling? Not us, right?
It's this understanding of electron behavior that allows chemists to predict and create new substances. It's like having a secret decoder ring for the universe! And for calcium and iodine, the code is CaI₂. Keep that little gem in your mind; it's a classic. It's a fundamental building block of our understanding of how elements interact. So, there you have it. The best formula for reacting elements calcium and iodine, explained over a presumably delicious beverage. Cheers to chemistry!
