Determine At What Temperature The Following Reaction Is Spontaneous
Ever stared at a chemical reaction and wondered, "When does this party actually start?" Like, when does it decide to go from "meh" to "WOOHOO, let's do this!"? Well, my friends, we're diving into the thrilling world of spontaneity. And no, it's not about spontaneously breaking into song (though that's fun too). We're talking about chemical reactions.
So, what exactly makes a reaction spontaneous? It's not about if it's fast or slow. A super slow reaction can totally be spontaneous. Think of rust. It takes ages, but it's happening all on its own! Spontaneity is all about whether a reaction wants to happen. It's like a shy introverted molecule finally deciding to mingle at the chemical party.
To figure out when our little chemical buddies decide to get busy, we need to look at a few key players. It's like trying to figure out if a date is going to be a hit. You gotta consider a couple of things!
The Energy Vibe: Enthalpy (ΔH)
First up, we have enthalpy, or ΔH. This is basically the heat of the reaction. Is it giving off heat (exothermic, negative ΔH) or sucking it up (endothermic, positive ΔH)? Think of it like this: reactions that give off heat are often super chill. They're like, "Here's some energy, have fun!" These guys are generally more inclined to be spontaneous. It’s like a good conversation that just flows – energy is released, and everyone feels good. A negative ΔH is your friend for spontaneity!
On the flip side, if a reaction needs a bunch of energy just to get going (positive ΔH), it’s a bit more of a high-maintenance guest. It’s like trying to get a cat to sit on your lap – it’s gonna need some serious persuasion. While not impossible, high energy costs can make a reaction less keen to spontaneously join the fun.
The Messiness Factor: Entropy (ΔS)
Next, we have entropy, or ΔS. This is where things get really fun. Entropy is basically a measure of disorder or randomness. Is the reaction creating more chaos or trying to tidy up? Nature, in its infinite wisdom, loves messiness. So, reactions that increase disorder (positive ΔS) are generally favored. Think of your bedroom after a week of intense studying. It's probably not spontaneously tidying itself up, right? That's entropy in action!
So, if a reaction goes from a neat, ordered crystal to a chaotic gas, that’s a big win for spontaneity! More moles of gas? More random movement? YES. That’s what we like to see. A positive ΔS is like the universe saying, "Go forth and be messy!" It’s a driving force for things to just... happen.

The Secret Sauce: Gibbs Free Energy (ΔG)
Now for the main event, the ultimate predictor: Gibbs free energy, or ΔG. This is where enthalpy and entropy have a little chat and decide the fate of our reaction. The equation is super neat:
ΔG = ΔH - TΔS
See that 'T'? That's temperature! This is where our question comes in. The temperature plays a HUGE role in whether a reaction throws a spontaneous party.
For a reaction to be spontaneous, ΔG needs to be negative. That's the magic number. It's like the VIP pass to the spontaneous reaction club.
Let's break down how temperature, represented by 'T', messes with this equation:
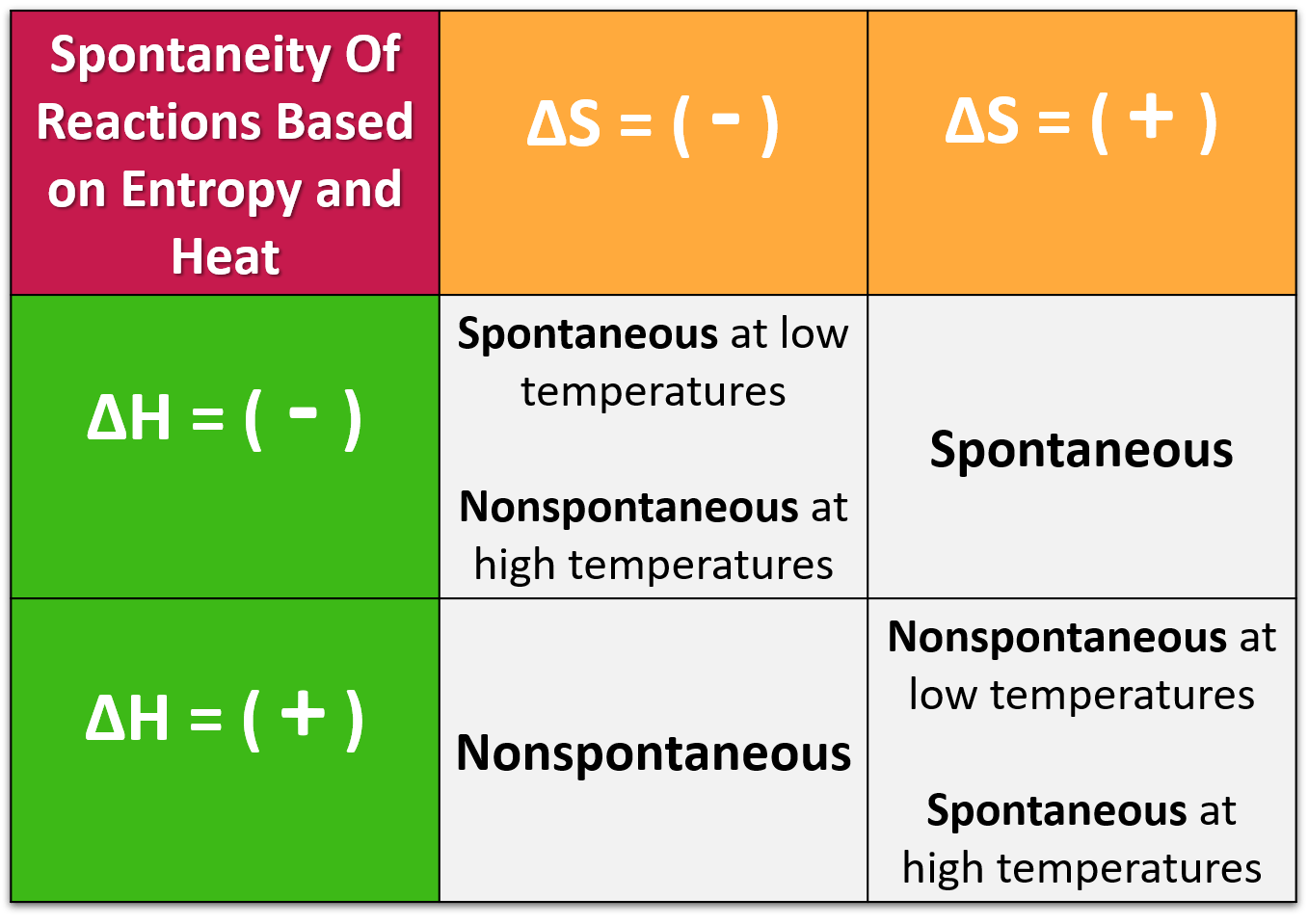
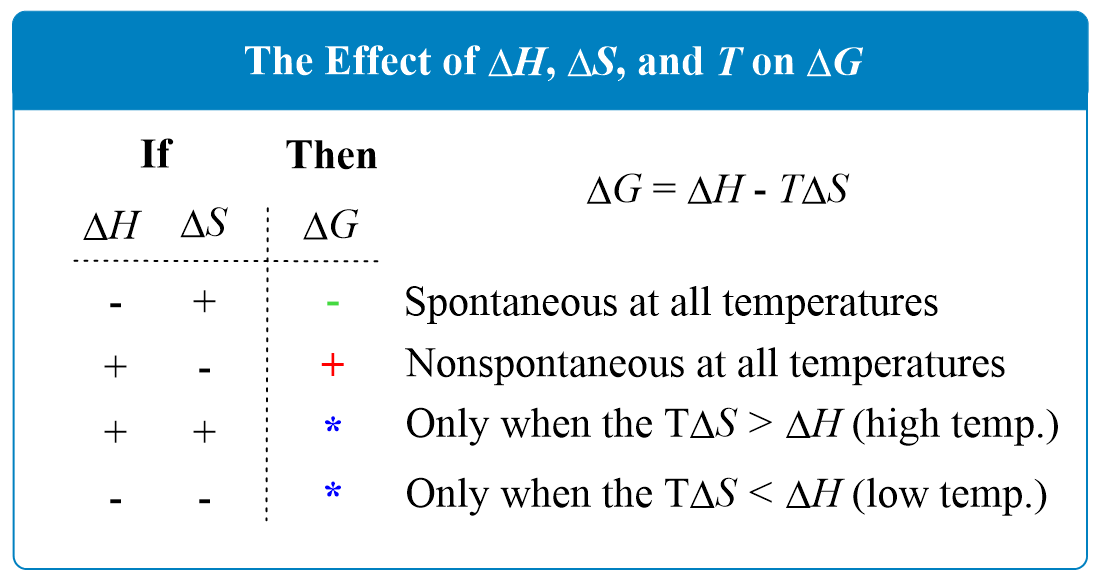
Scenario 1: You're a Chill Reaction (Negative ΔH) and a Messy Maker (Positive ΔS)
This is the dream team! You're already giving off energy AND increasing disorder. You're basically a chemical superhero. In this case, ΔH is negative, and -TΔS will also be negative (since T is always positive). So, ΔG will always be negative, no matter the temperature. This reaction is spontaneous at ALL temperatures. It's like a perfectly ripe avocado – ready to go whenever!
Scenario 2: You're a High-Maintenance Energy Guzzler (Positive ΔH) but a Super Messy Maker (Positive ΔS)
Okay, this one is interesting. ΔH is positive, but -TΔS is negative. So, ΔG = (positive) - T * (positive). If the temperature (T) is low, the TΔS term will be small. You might have a positive ΔG, meaning non-spontaneous. BUT! As you crank up the temperature (T), the -TΔS term gets bigger and more negative. Eventually, it'll be big enough to outweigh the positive ΔH. Poof! ΔG becomes negative, and the reaction becomes spontaneous at HIGH temperatures. Think of boiling water. It takes energy (positive ΔH), but as it gets hotter, it becomes a chaotic gas (positive ΔS) and is spontaneous. It’s like a difficult puzzle that eventually becomes clear and enjoyable as you spend more time on it.
Scenario 3: You're a Chill Energy Giver (Negative ΔH) but a Neat Freak (Negative ΔS)
This is the opposite of scenario 2. ΔH is negative, and -TΔS is positive (because ΔS is negative). So, ΔG = (negative) - T * (negative) = (negative) + T * (positive). At low temperatures, the negative ΔH will dominate, making ΔG negative. Spontaneous! But as you increase the temperature (T), the positive TΔS term starts to grow. Eventually, it will be big enough to cancel out the negative ΔH. ΔG becomes positive, and the reaction is no longer spontaneous. This reaction is spontaneous at LOW temperatures. It's like a shy person who is comfortable in a small, quiet gathering but gets overwhelmed at a huge party.

Scenario 4: You're a High-Energy Guzzler (Positive ΔH) and a Neat Freak (Negative ΔS)
Uh oh. This is the chemical equivalent of a bad hair day. ΔH is positive, and -TΔS is positive. So, ΔG = (positive) + T * (positive). No matter what temperature you try, ΔG will always be positive. This reaction is NEVER spontaneous at any temperature. It’s like trying to push a boulder uphill in the rain – it’s just not going to happen on its own.
So, to answer the big question: "At What Temperature Is This Reaction Spontaneous?"
You gotta look at the ΔH and ΔS values for the specific reaction you're interested in. Once you have those numbers, you plug them into the equation ΔG = ΔH - TΔS and see where ΔG crosses the zero line.
If ΔG = 0, that’s the exact temperature where the reaction is at equilibrium. It’s not spontaneous in either direction. It’s just… hanging out. The point where it transitions from non-spontaneous to spontaneous is when ΔG goes from positive to negative.
You can even rearrange the equation to find that magic temperature:

At equilibrium, ΔG = 0, so 0 = ΔH - TΔS
Rearranging gives us: T = ΔH / ΔS
This 'T' is the transition temperature. If your reaction is spontaneous at high temperatures (Scenario 2), then any temperature above this T is spontaneous. If it’s spontaneous at low temperatures (Scenario 3), then any temperature below this T is spontaneous.
It's like tuning a radio! You're trying to find that perfect frequency where the music (spontaneity) kicks in. Chemistry isn't just about boring equations; it's about understanding the driving forces behind everything that happens, from a tiny spark to the grandest cosmic event. Pretty cool, right?
