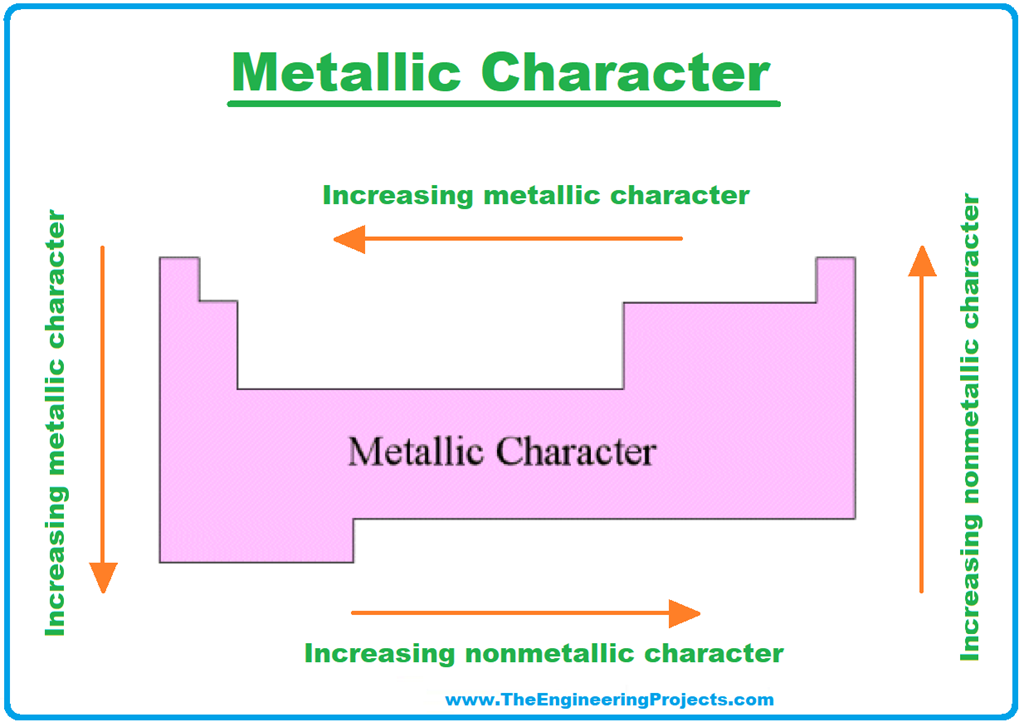
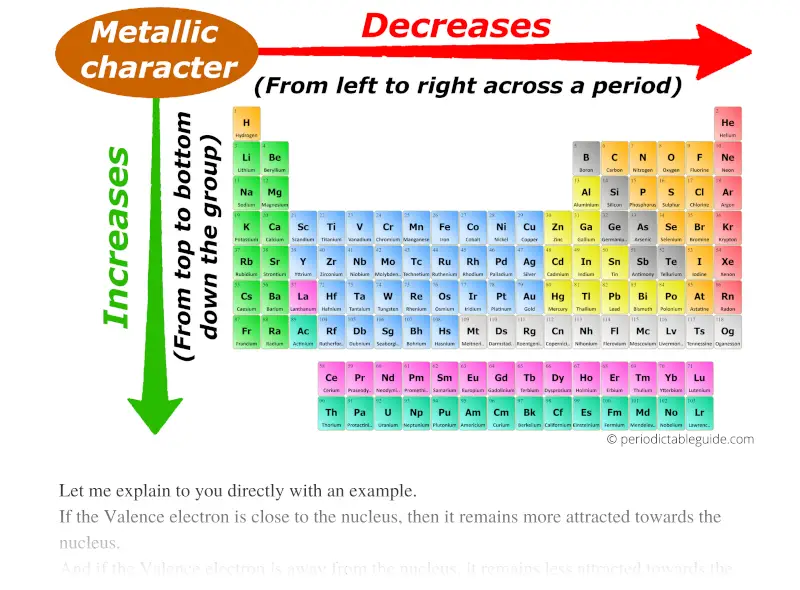
Describe The Trend In Metallic Character Going Down A Group

Hey there, science explorers! Ever looked at a periodic table and wondered what’s up with all those numbers and symbols? It’s like a secret code for the universe, right? Well, today we’re going to peek behind the curtain and talk about something pretty neat: metallic character and how it dances its way down the periodic table's columns, or as we cool cats call them, groups.
So, what even is metallic character? Think of it like this: metals are generally known for being shiny, good at conducting electricity and heat, and, importantly for our chat today, they tend to give away electrons. They’re like the generous friends of the chemical world, always happy to share their outer electrons.
Now, imagine you're going down a group in the periodic table. It’s like descending into a cozy, well-stocked basement. What changes as you go deeper?
The Downhill Slide of "Metally-ness"
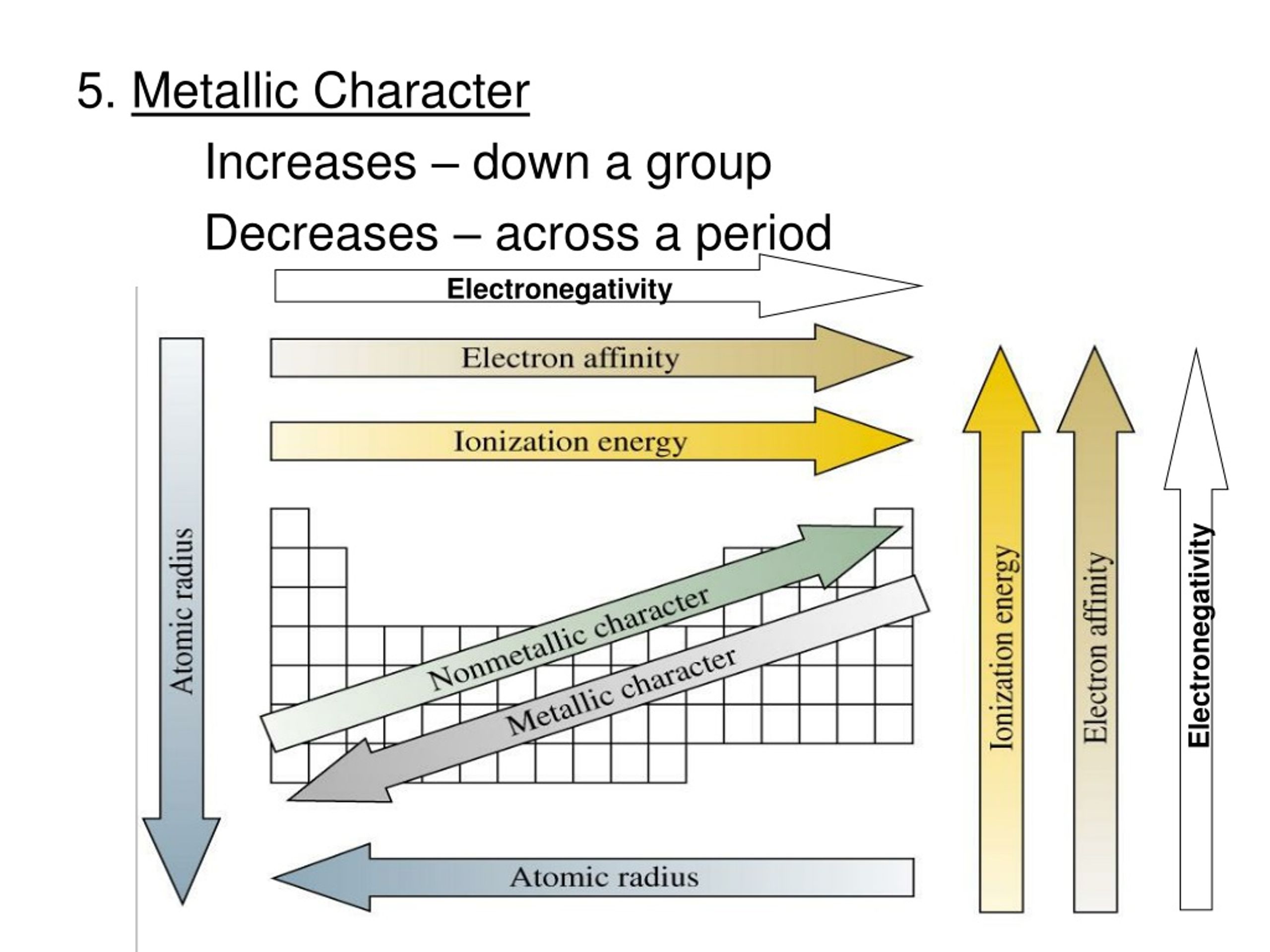
It might sound a bit counterintuitive at first, but as you move down a group, the metallic character actually increases. Yep, you heard that right! The elements get more metallic as you go deeper. So, if you're thinking of a group as a ladder, the metallic character is like the grip getting stronger and stronger the further down you climb.
But why? That’s where the real magic happens, and it all boils down to the electrons. You know those tiny little particles that orbit the nucleus of an atom? They’re like little planets around a sun.
As you descend a group, each new element has an extra electron shell. Think of it like adding another floor to a building. So, the outermost electrons, the ones involved in all the chemical bonding fun, are now further away from the center of the atom, the nucleus.
Imagine you're trying to hold onto a balloon. If you’re holding it right next to you, it’s easy to keep a firm grip. But if you stretch your arm out really far, that balloon is much easier to pull away, right? It’s kind of similar with these electrons.
The nucleus has a positive charge, and the electrons have a negative charge. They’re attracted to each other, like little magnets. But when those outer electrons are further away, that attraction isn't as strong. The pull from the nucleus is weakened.
The Nucleus's Losing Grip
This weaker attraction means it's easier for the atom to let go of those outer electrons. And remember, letting go of electrons? That's the hallmark of a metal! The more readily an atom can give up its electrons, the more metallic it is.
So, as we go down a group:
- More electron shells = outer electrons are further from the nucleus.
- Weaker attraction between the nucleus and outer electrons.
- Easier to lose electrons.
- Higher metallic character!
Think of it like this: the top element in a group might be a bit shy about giving away its electrons. It holds onto them pretty tightly. But as you go down, the atoms become more and more eager to shed those outer electrons, becoming super cooperative and, well, more metallic.
Let's Meet Some Real-Life Examples!
Let’s take Group 1, the alkali metals, as our superstar example. At the top, we have Lithium (Li). It’s a metal, for sure, but it’s not as reactive as the ones lower down.
Then we have Sodium (Na) – you know, the stuff in table salt (NaCl). Sodium is quite reactive, eager to give up its electron. It’s definitely more metallic than Lithium.

Keep going down, and you hit Potassium (K). Potassium is even more reactive and more metallic than Sodium. It’s like the trend is accelerating!
And then there's Rubidium (Rb) and Cesium (Cs). By the time you get to Cesium, you're dealing with an element that is incredibly reactive and readily gives up its electrons. It’s practically begging to be ionized! Cesium is way more metallic than Lithium.
It's like comparing a tiny sprout to a giant redwood tree. Both are plants, but their scale and influence are vastly different. Similarly, both Lithium and Cesium are metals, but Cesium exhibits a much stronger metallic character due to its larger size and looser hold on its electrons.
The Atomic Radius Connection
This concept is also closely tied to the atomic radius. As you go down a group, the atoms get bigger. It’s like adding rings to a tree trunk – each new shell adds to the overall diameter. Bigger atoms mean those outer electrons are even further from the nucleus, reinforcing that weaker attraction and making them easier to remove. So, larger atomic radius often goes hand-in-hand with greater metallic character.
Why Does This Matter? It's Pretty Cool, Actually!
So, why should we care about this trend? Well, understanding how metallic character changes helps us predict how elements will behave in chemical reactions. If we know an element is highly metallic, we can expect it to be a strong reducing agent – meaning it’s good at causing other things to be reduced (by giving away its own electrons).
It also helps us understand the properties of materials. The metallic character of elements influences everything from the conductivity of wires to the reactivity of batteries. It’s the fundamental stuff that makes our technological world possible.
Plus, it’s just fascinating to see how these tiny, fundamental building blocks of the universe follow such predictable patterns. It's like a cosmic choreography!
So, the next time you glance at the periodic table, remember the journey down those groups. It’s not just a random arrangement; it’s a map showing how the elemental personalities change, with metallic character becoming ever more pronounced as you descend. It’s a simple, yet profound, dance of electrons and atomic structure that underpins so much of chemistry!
