Deducing The Block Of An Element From An Electron Configuration

Alright folks, gather 'round, grab your artisanal coffee, and let's dive into the wild, wonderful world of electrons. You know, those tiny little troublemakers that zip around an atom's nucleus like a caffeinated squirrel on roller skates? Well, turns out, understanding their chaotic dance can actually tell us something super cool: which block of the periodic table our element calls home. Think of it as finding its ZIP code, but way more… electrifying!
Now, the periodic table. It’s not just a fancy wall poster for nerds (though we love our nerds!). It’s actually organized with a brilliant, almost sinister, logic. And this logic, my friends, is built on electron configurations. Specifically, it’s all about where that last electron decides to crash for the night. It’s like the ultimate electron slumber party, and the location of that final guest dictates everything.
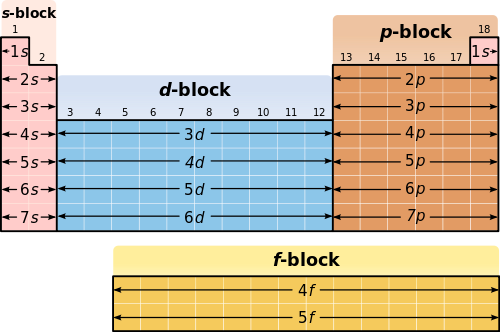
So, what are these mystical "blocks"? Imagine the periodic table as a giant apartment building. We’ve got the S-block, the P-block, the D-block, and the F-block. Each block is like a different wing of the building, with its own vibe and, more importantly, its own type of electron orbital where the last electron hangs out. It’s a bit like choosing between a minimalist studio (S-block), a cozy one-bedroom with a balcony (P-block), a sprawling loft (D-block), or a super-exclusive penthouse suite (F-block).
The S-Block: The Chill Neighbors
Let's start with the S-block. These guys are like the chill, easy-going neighbors. They’re the first two columns of the periodic table – Hydrogen, Helium, Lithium, Beryllium, and so on. Their electron configurations are super straightforward. The last electron they snag always goes into an ‘s’ orbital. It’s like they’ve got this one, really comfortable couch in their living room, and everyone just gravitates towards it.
Think about Lithium, Li. Its electron configuration is 1s²2s¹. See that ‘2s¹’? That ‘s’ at the end? Bingo! That tells you it’s an S-block element. The ‘2’ just means it’s in the second energy level, which is like saying the apartment is on the second floor. But the ‘s’ is the key – it’s heading for the ‘s’ orbital, the S-block’s favorite hangout spot.
And Helium? It’s a bit of an oddball. Its configuration is 1s². It’s technically in the S-block, even though it behaves like a noble gas. It’s like that one person who lives in a studio apartment but insists on having a massive, king-sized bed. Go figure!
The P-Block: The Creative Types
Moving on to the P-block! This is where things get a little more… diverse. These are the columns from Aluminum to Argon, and so on. If your element's last electron decides to chill in a ‘p’ orbital, congratulations, you’re in the P-block! These are the artistic types, the thinkers, the ones who are always adding a little extra flair.
For example, Nitrogen, N. Its configuration ends in 2p³. That ‘p’ is your golden ticket to the P-block. The ‘2’ again signifies the energy level (the second floor, if you will), and the ‘p³’ means it’s got three electrons happily settled into the p orbitals. P orbitals are a bit more complex than s orbitals – they’re shaped like dumbbells, almost like tiny little infinity symbols. Very artsy, right?
This is where you find all sorts of characters: the metals, the nonmetals, and those fascinating metalloids in between. It’s the P-block where the real action happens in terms of chemical reactions for many common elements. They’re the bustling downtown of the periodic table.
The D-Block: The Trendsetters (and the Heavyweights!)
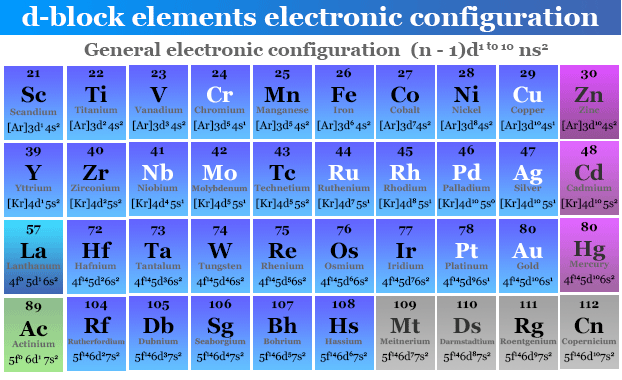
Now, let’s talk about the D-block. These are the transition metals, the ones in the middle of the periodic table that look all shiny and sophisticated. If your element’s last electron is destined for a ‘d’ orbital, welcome to the D-block party!

Think Iron, Fe. Its electron configuration is a bit longer, but the crucial part often ends with something like 3d⁶. See that ‘d’? That’s your D-block indicator. The ‘3’ here refers to the third to last energy level. It’s a bit like they’re packing for a trip, and the last electron is deciding which suitcase to put in the second to last spot on the plane. Confusing? A little. But it’s how the universe likes to keep us on our toes!
D orbitals are even more complex, shaped like clover leaves or weird balloons. They’re where the magic of metallic bonding really shines. These are the elements that conduct electricity like nobody’s business, often have multiple oxidation states (meaning they can be multiple personalities in a chemical reaction – talk about commitment issues!), and are generally the heavyweights of the chemical world. They’re the cool kids with the fancy cars and the complicated hairdos.
The F-Block: The Elusive Elites
Finally, we have the F-block. These are the lanthanides and actinides, usually tucked away at the very bottom of the periodic table like a secret VIP lounge. If your element’s last electron is heading into an ‘f’ orbital, you’ve found yourself an F-block element. These are the rare earths, the radioactive guys, the elements that make scientists go “Ooooooh!”
Take Uranium, U. Its electron configuration gets seriously long, but it will eventually have an electron in an ‘f’ orbital, like 5f³. The ‘5’ here is again referring to the fifth energy level from the end. And the ‘f’ orbital? It’s so complex, it looks like a mishmash of even more lobes and shapes. They’re the ultimate show-offs of the orbital world.
These elements are often incredibly reactive or have really specific uses in high-tech industries and, well, nuclear power. They’re the reclusive geniuses, the ones who don’t play well with others but are incredibly powerful when they do. They’re like the eccentric billionaires of the periodic table – fascinating, a bit dangerous, and definitely not for everyone.
Putting It All Together: Your Electron Detective Kit
So, how do you put this into practice? It’s all about looking at that electron configuration and asking yourself: “Where did that last electron park its tiny little electron behind?”
If the last electron is in an s orbital, it’s S-block.
If the last electron is in a p orbital, it’s P-block.
If the last electron is in a d orbital, it’s D-block.
If the last electron is in an f orbital, it’s F-block.
It’s like having a secret decoder ring for the periodic table! You don’t need a degree in rocket science; you just need to know your orbitals. So next time you see an electron configuration, don’t just see a string of numbers and letters. See the story, see the neighborhood, see the block!
And that, my friends, is how you deduce the block of an element from its electron configuration. Pretty neat, huh? Now, who wants another coffee? This electron-chasing business is exhausting!
