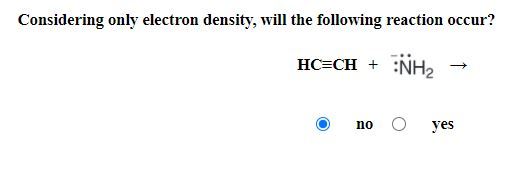
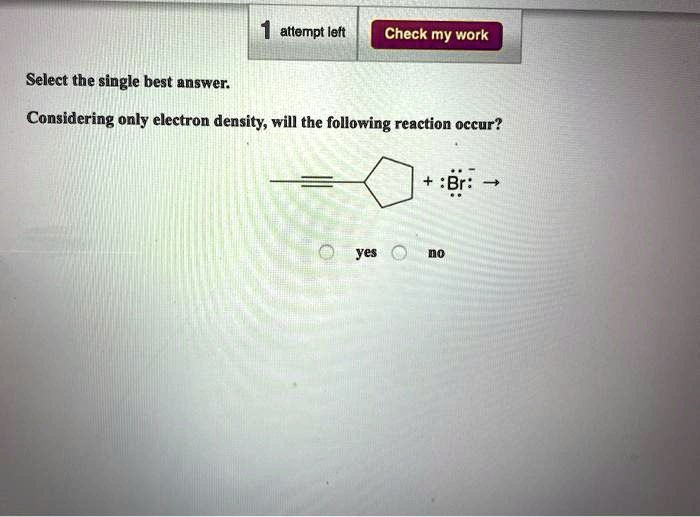
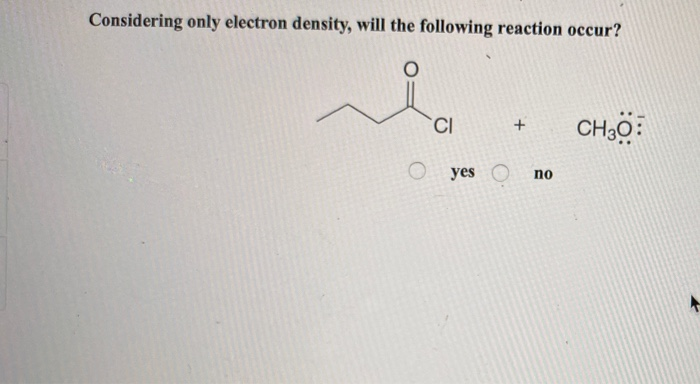
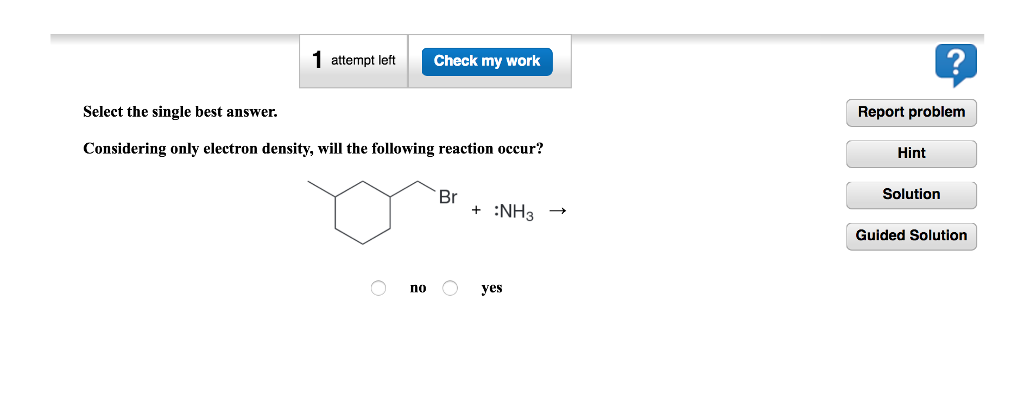
Considering Only Electron Density Will The Following Reaction Occur
Have you ever wondered about the secret lives of molecules? They’re like tiny, invisible dancers, always moving and interacting. Sometimes, these dances lead to something new and exciting – a chemical reaction!
But what makes these molecular parties start? It's not always about fireworks and loud music. Often, it's a much subtler thing that gets the ball rolling.
Imagine you’re trying to figure out if two people will get along. You could look at their personalities, their interests, or maybe even how much they like the same snacks. In the world of chemistry, we have our own special way of peeking into the lives of molecules.
This way involves looking at something called electron density. Now, don't let the fancy name scare you! Think of it like this: electrons are the super energetic little bits that zip around the outside of atoms. Electron density is basically a map of where these busy electrons like to hang out the most.
It's kind of like knowing where all the popular spots are in a town. If you know where the most people (electrons) gather, you might get a good idea of what's going on.
Now, here’s where it gets really fun and a little bit mind-bending. We're going to ask a big question: if we only look at this map of electron density, can we predict if a chemical reaction will happen? Can we say, “Yep, based on where these electrons are chilling, a change is definitely going to occur!”?
It’s like trying to guess if a party will happen just by looking at the blueprints of a house. You can see the rooms, the windows, and the doors, but can you tell if there will be laughter and dancing inside?
This is where our adventure begins. We’re not going to consider all the usual suspects that chemists talk about – like energy levels, or how stable things are, or even the temperature. Nope, we're stripping it all back. We're going to focus solely on the distribution of these tiny, buzzing electrons.
Is it possible to be a detective and solve the mystery of a chemical reaction using just one clue? That's the tantalizing question we're exploring. It’s a bit like a puzzle where you’re given only a corner piece and asked to imagine the whole picture.
And let me tell you, when you start to dive into this, things get surprisingly interesting. You begin to see the world of chemistry in a completely new light. It’s like putting on special glasses that let you see the invisible forces at play.
Think about it. Molecules are constantly bumping into each other. Sometimes, these bumps are just casual greetings. Other times, they’re the sparks that ignite a whole new formation.
What if the places where electrons are super concentrated, like bustling neighborhoods, are the key to understanding these transformations? What if the areas where electrons are scarce, like quiet empty lots, also tell a story?
This isn't your typical textbook explanation. We’re going on a bit of a quest. A quest to understand the fundamental drivers of chemical change. And we’re doing it with a rather… minimalist approach.
It’s like asking a chef to create a masterpiece using only salt and pepper. Can they do it? What kind of flavors can they coax out with just those two elements? It’s a challenge that forces you to think creatively.
So, imagine we have a specific reaction in mind. We’re going to zoom in on the molecules involved. We're going to draw up detailed maps of their electron density. We’re going to meticulously examine where the electrons are densely packed and where they are sparse.
And then, based only on these maps, we’re going to make a prediction. Will these molecules decide to dance together and form something new? Or will they just politely wave and move on?
This is where the suspense builds. You're looking at these beautiful, intricate patterns of electron distribution. You're trying to find the clues. It’s a bit like a treasure hunt, but instead of gold, you’re looking for the tell-tale signs of chemical reactivity.
What makes this so special? Well, it’s the elegance of it. In science, we often have complex theories and lots of factors to consider. But sometimes, the most profound insights come from looking at the simplest, most fundamental aspects.
And electron density? It's one of the most fundamental properties of a molecule. It tells you where the "stuff" of the molecule is concentrated. It’s the electronic "heartbeat" of an atom or molecule.
So, when we focus only on this, we’re essentially stripping away all the other noise. We’re trying to see if the core electron distribution itself holds enough information to predict the outcome. It’s a pure, unadulterated look at molecular behavior.
This is why it’s so engaging. It’s a challenge to our intuition. It makes us question our assumptions. It forces us to be really precise in our observations.
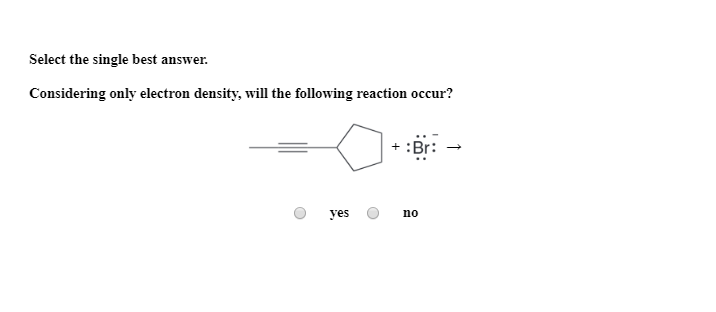
Think of a perfectly balanced scale. If you add just a tiny bit more weight to one side, the whole thing tips. In a reaction, it’s often a similar subtle shift in where electrons are that can make all the difference.
Can we, with just this electron map, predict which way the scale will tip? Can we see the subtle imbalances that will lead to a new arrangement? It’s a thrilling prospect.
And when you start to look at actual examples, it’s fascinating. You see molecules with certain electron-rich areas. You see others with electron-poor pockets. And you start to connect these patterns to whether a reaction actually occurs or not.
It's like having a secret handshake. If the electrons are in the right "places" and have the right "density" in those places, they might just be signalling to each other, "Hey, let's react!"
This approach is incredibly powerful because it gets to the heart of why chemical bonds form and break. These bonds are all about the sharing and redistribution of electrons. So, understanding where those electrons are is like understanding the very fabric of chemical change.
It's also a testament to how much information can be packed into seemingly simple concepts. We often think of electrons as just tiny particles, but their distribution, their density, tells a complex story.
It’s a bit like looking at a cloud formation. You can see the shapes, the density of the water droplets. And just by looking at that, a meteorologist can predict if it’s going to rain. They’re not necessarily looking at the temperature of the ground, or the wind speed a thousand miles away. They're focusing on the immediate visual clues.
Similarly, by focusing on electron density, we’re looking at the immediate visual clues of the molecules themselves. We’re saying, "What can the molecules tell us just by how their electrons are arranged?"
This is what makes it so special and so entertaining. It’s a puzzle, a detective story, and a lesson in fundamental chemistry all rolled into one. It encourages us to look beyond the obvious and to appreciate the subtle forces that govern the world around us.
So, the next time you see a chemical reaction happening, remember the invisible dance of electrons. And consider how much information is hidden within their density. It might just be enough to tell the whole story.
This is a topic that will make you think. It will make you curious. And it might just inspire you to look at the world of chemistry with a new sense of wonder. It’s a peek into the fundamental language of molecules.
It’s a reminder that sometimes, the simplest observations can lead to the most profound discoveries. And that, my friends, is truly captivating. The universe of molecules is full of such hidden wonders, waiting to be uncovered by curious minds.
