Consider These Reactions Where M Represents A Generic Metal
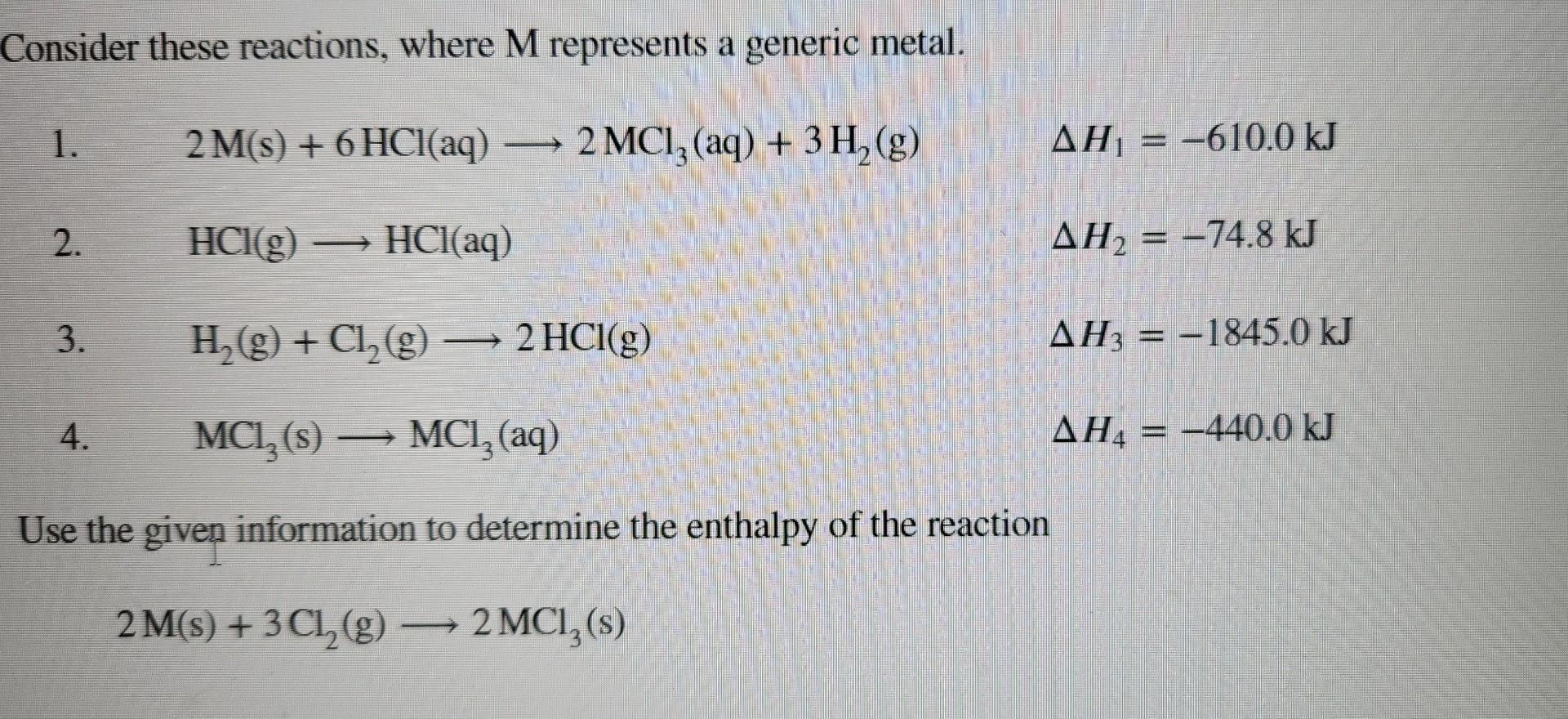
Have you ever wondered what happens when you mix different substances together? It's a bit like a culinary experiment, but instead of tasting delicious food, we're observing fascinating chemical changes. Today, let's dip our toes into the intriguing world of chemical reactions, specifically those involving a generic metal, which we'll represent with the letter M. It's not as complicated as it sounds, and understanding these basic reactions can be surprisingly fun and illuminating, giving us a glimpse into the hidden workings of the world around us.
Why bother with generic metals and reactions? Well, these reactions are the building blocks of so much. They explain why metals rust, how batteries work, and even how our bodies process nutrients. Understanding these fundamental processes helps us appreciate the science behind everyday phenomena. The primary purpose of studying reactions like those involving M is to learn about how matter transforms. The benefits are immense: improved understanding of materials, development of new technologies, and even safer handling of chemicals. It’s about uncovering the why and how behind chemical change.
You might be surprised to learn where these concepts pop up. In education, they are foundational. Students encounter them in chemistry classes, learning to predict what might happen when M reacts with, say, an acid (think of a fizzing antacid tablet – that's a metal-like substance reacting with acid!). In daily life, consider the corrosion of a metal railing on your porch. That's a reaction, often involving oxygen and moisture, where the metal M changes into a different compound, usually an oxide. Another example is the production of cleaning agents, where specific reactions are carefully controlled to create effective solutions.
So, how can you explore this curiosity yourself, without a full lab setup? It's simpler than you might think! One easy way is to observe common reactions. Notice how copper pennies tarnish over time – that's a slow reaction with the air. You can also experiment with household items, with adult supervision, of course. For instance, mixing baking soda (a compound containing sodium, a metal-like element) with vinegar (an acid) creates a satisfying fizz. While not a direct M reaction, it demonstrates the principle of different substances interacting. You can also look for educational videos online that visually demonstrate these reactions in a safe and engaging way. These visual aids can make the abstract concepts much more concrete. Essentially, just being more observant of the chemical transformations happening around you is a great starting point!
