Consider The Structure Of Sucrose With Labeled Carbon Atoms

Alright, so picture this: you're at a fancy tea party, right? And there's a little plate of sugar cubes, all pristine and innocent. You pop one in your tea, thinking, "Ah, sweet bliss." But have you ever stopped to wonder what’s really going on in that tiny, crystalline cube? I'm talking about sucrose, my friends. That’s right, the fancy name for the stuff that makes your coffee bearable and your cookies sing. And today, we're going to get a little… nerdy about it. But don't worry, it's the fun kind of nerdy, the kind where we're not dissecting frogs, we're dissecting sugar. So, grab your imaginary teacup, and let's dive into the wonderfully weird world of labeled carbon atoms in sucrose.
Now, sucrose isn't just some amorphous blob of sweetness. Oh no. It's a molecule with a seriously organized structure. Think of it like a molecular condo complex, with different rooms and residents. And the VIP residents? Those are our carbon atoms. They're the building blocks, the architects, the little dudes holding the whole sweet party together. And here's where it gets really interesting: we can actually give these carbon atoms little name tags, or, in science-speak, we can label them. It's like putting tiny little GPS trackers on them so we know exactly where they're hanging out and what mischief they're getting up to.
The Two-Room Sweet Suite
So, sucrose, this sugar superstar, is actually made up of two smaller sugars that have decided to get hitched. It’s a bit like a arranged marriage in the molecular world, but a very successful one! We have glucose and fructose. They're like the dynamic duo of sweetness, and they've joined forces to create this magnificent sucrose molecule. Think of glucose as the more stable, perhaps slightly more buttoned-up cousin, and fructose as the wilder, fruitier sibling. When they link up, it's pure, unadulterated sweetness magic.
Now, each of these little sugar individuals – glucose and fructose – has its own set of carbon atoms. And the way they’re arranged, the number of carbons they have, and where the oxygen and hydrogen atoms are attached, that’s what gives them their unique personalities and makes them… well, glucose and fructose. It’s like having two different Lego sets, and when you snap them together in a very specific way, BAM! You get sucrose.
Let's Get Personal: Tagging Our Carbon Buddies
Okay, here's where the labeling fun begins. Imagine we're throwing a party for our carbon atoms in the glucose part of sucrose. We decide to mark certain carbons with a special sticker. Let’s say we’re talking about glucose first. It’s got six carbons, right? We can label them Carbon-1, Carbon-2, all the way up to Carbon-6. It's a bit like assigning roles in a play: "Okay, Carbon-1, you're the opening line. Carbon-2, you're the dramatic pause."
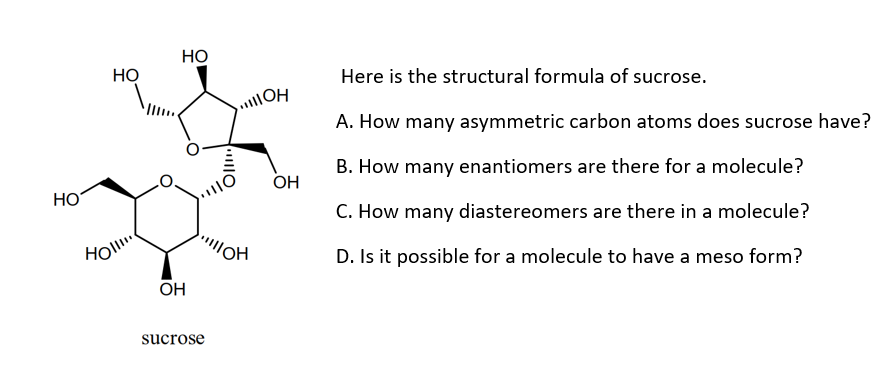
So, if we were to label the carbons in glucose, we might say, "Hey, let's pay special attention to Carbon-1 of glucose." This guy is really important because it's where the action happens when glucose decides to bond with its fructose partner. It's the handshake point, the "nice to meet you" spot. And then there's Carbon-2 of glucose, which is busy being all chemically charged up, ready for reactions. And so on, each carbon having its own little job description.
Now, let's hop over to the fructose side of the molecular mansion. Fructose is a bit different. It's a five-membered ring (or sometimes a six-membered one, but let's keep it simple for now!), and it also has six carbons, but they're arranged a little differently. So, when we label the carbons in fructose, we’re essentially starting a new numbering system. We've got Carbon-1 of fructose, Carbon-2 of fructose, and so on. It's like moving to a new neighborhood – you've got the same types of houses, but the street numbers might be different.
The critical connection point between glucose and fructose in sucrose happens between Carbon-1 of glucose and Carbon-2 of fructose. This is the epicentre of their sweet union! It’s where they link hands, forming the glycosidic bond that holds them together. Think of it like a molecular high-five that lasts forever (or until you decide to put that sugar cube in your tea and your saliva starts doing its thing). And this is where the magic of labeling really shines. By tracking which carbon from glucose connects to which carbon from fructose, scientists can understand how these molecules are built and broken down.
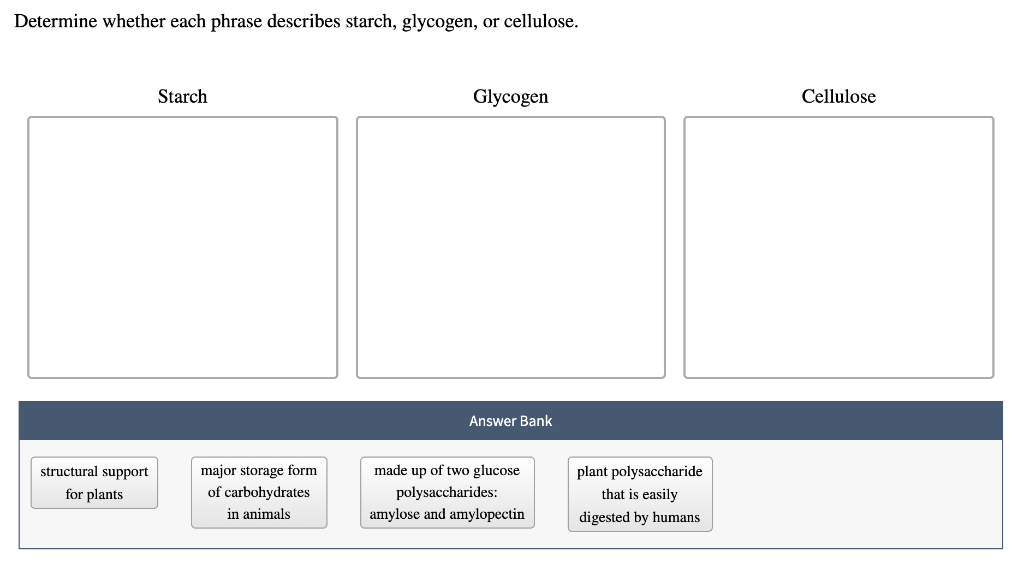
Why Bother With Tiny Name Tags?
You might be thinking, "Why are we obsessing over these tiny carbon name tags? What’s the big deal?" Well, my friends, it's a HUGE deal! Imagine you're trying to figure out how a complex machine works. You can't just look at the whole thing; you need to understand how each little gear and spring interacts. Labeling carbon atoms is exactly like that for molecules. It allows scientists to trace the path of atoms during chemical reactions.
For instance, when our bodies digest sucrose, it’s like a molecular demolition crew comes in. Enzymes, those little biological workers, chop up the sucrose molecule. By labeling the carbons, scientists can follow exactly which pieces of glucose and fructose end up where in our bodies, and how they’re used for energy. It's like watching a breadcrumb trail of sweetness, but way more scientifically significant.
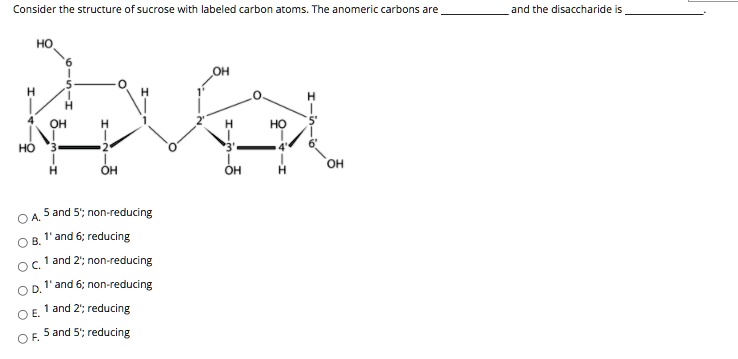
It also helps us understand how we make things. If we're trying to synthesize sucrose in a lab, or understand how plants make it in the first place (which is a whole other fascinating story involving photosynthesis and sunshine – talk about natural powerhouses!), labeling allows us to confirm that we're building the molecule correctly. We can say, "Yep, Carbon-1 of glucose is definitely connected to Carbon-2 of fructose, just like the recipe says!"
And here’s a little surprising fact for you: plants actually make sucrose as their primary transportable sugar. They're like little sugar factories, churning it out to send energy all over the place. And when they need to store energy, they often convert it into starch. So, that potato you’re eating? It’s got a whole history of sucrose transformations going on inside!
So, the next time you sprinkle sugar on your cereal or stir it into your coffee, take a moment to appreciate the intricate world of sucrose. It's not just sweetness; it's a beautifully constructed molecule with labeled carbon atoms, each playing a crucial role in its existence and our enjoyment. It’s a tiny, sweet testament to the incredible order and complexity of the universe, all packed into a single grain of sugar. Pretty wild, right? Now, if you'll excuse me, I think I need another cup of tea. For science, of course.
