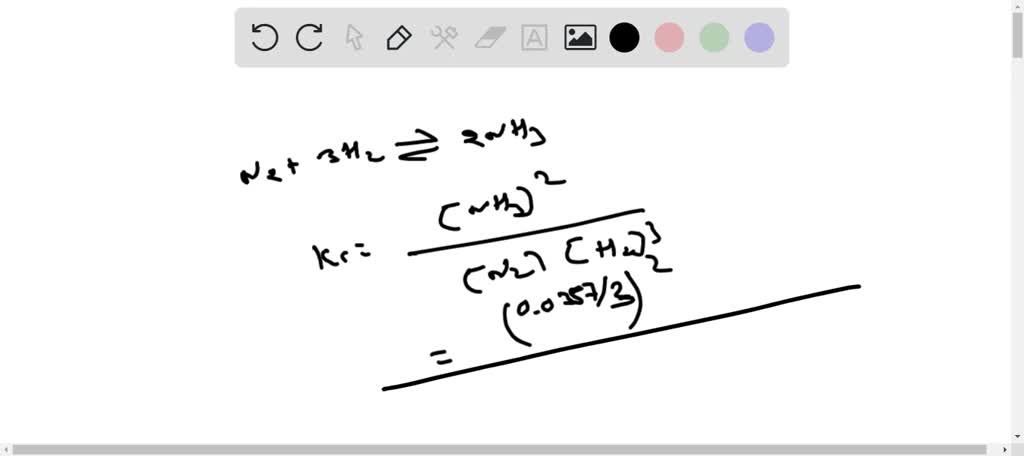
Consider The Following Reversible Reaction. In A 3.00-liter Container
Have you ever seen something that looks like it's going in two directions at once? It's like a magic trick, but it's actually chemistry! We're talking about a reversible reaction. Think of it like a perfectly balanced seesaw. On one side, things are getting made. On the other side, those very same things are breaking back down.
Imagine a busy marketplace. People are constantly buying goods (making new things) and then, maybe they decide to return those goods (breaking them back down). It's a constant flow, back and forth. That's kind of what's happening in this special little experiment we're going to talk about. We've got a 3.00-liter container. That might sound like a specific number, but it just gives us a place to play. It's like a stage for our tiny chemical actors.
So, what's so entertaining about this whole reversible reaction thing? Well, it's all about balance. Imagine you have a bunch of kids playing a game. Some are on one team, some on the other. They're all running around, switching sides. At some point, you might notice that the number of kids on each team stays pretty much the same, even though individuals are constantly moving. That's called equilibrium, and it's the star of this show!
In our reversible reaction, the molecules are like those kids. They're zipping around, bumping into each other. Some of them are joining up to form new molecules. That's the "forward reaction." But then, these new molecules can also break apart again, turning back into what they were before. That's the "reverse reaction." It's a never-ending dance of creation and deconstruction, all happening in our tidy 3.00-liter container.
What makes it so special is the idea that it's not a one-way street. Most reactions, you think of them as going to completion. Like baking a cake – once it's baked, it's done. You don't usually un-bake a cake, right? But with a reversible reaction, it’s like having a cake that can un-bake itself and become batter again, and then bake itself into a cake, and on and on.

The magic really kicks in when things reach equilibrium. It's not that the reaction stops. Oh no! The forward and reverse reactions are still happening, at the exact same speed. It's like two people walking on treadmills facing each other. If they walk at the same speed, neither one actually moves forward or backward relative to the other. They're just in a constant, balanced motion.
Think about it: in that 3.00-liter container, these molecules are having a ball. They're making friends, then breaking up, then making new friends, and so on. It's a tiny, microscopic party! And the coolest part is that you can nudge this party. You can add more of one ingredient, or take some away, and the party will adjust itself to find a new balance. It's like adding a few more dancers to the floor – the existing dancers might shift around, but they'll find a new rhythm.
This concept of equilibrium is super important in tons of places. It's not just some abstract idea for science nerds. It's how your body works, how the environment stays balanced, and even how things are made in industries. So, when you hear about a reversible reaction in a 3.00-liter container, don't just think of boring chemicals. Think of a dynamic, lively system that's constantly seeking balance.

It's the constant give and take that's so fascinating. You have these reactants turning into products, and then these products turning back into reactants. It’s a beautifully messy, yet perfectly ordered, process. It’s like watching a tiny, never-ending movie where the plot is always unfolding, but also always resetting.
The fact that it doesn't just stop is what makes it so engaging. It's the idea that even when things look static, there's still a lot of activity going on beneath the surface. The 3.00-liter container becomes a miniature universe, governed by these elegant laws of chemistry. And the reversible reaction is the story it’s constantly telling.
So, next time you hear about a reversible reaction, especially one happening in a specific volume like a 3.00-liter container, picture that seesaw, that marketplace, that dance. Picture the molecules having a blast, constantly changing and yet, in a way, staying the same. It's a little bit of chemistry magic, right there in a box.
It makes you wonder, doesn't it? What exactly are these molecules? What are they becoming? And how do they manage to keep this whole thing going? It’s the mystery and the elegance of it all that truly draws you in. It’s a reminder that even in the smallest spaces, there’s incredible activity and a constant pursuit of harmony. And that, my friends, is what makes a reversible reaction so utterly entertaining and special.
Equilibrium is not the end, but a dynamic balance.
Isn't it neat to think that the world around us is full of these hidden, constantly shifting processes? The idea of a reversible reaction in a 3.00-liter container is just one example of the amazing dance of molecules that keeps our universe humming. It’s a little peek into a world where things are never truly still, but always finding their perfect, balanced rhythm.
