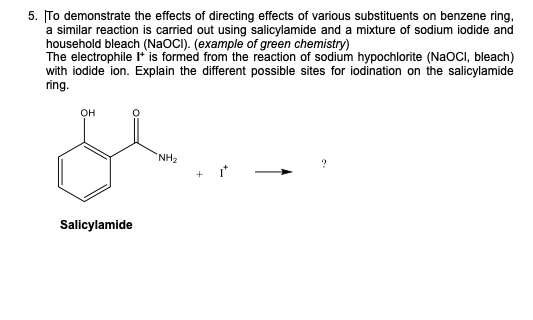
Consider The Directing Effects Of The Substituents On Salicylamide

Ever looked at a molecule and thought, "Man, this thing is going places!"? Well, buckle up, because we're diving into the totally awesome world of salicylamide and how its little buddies, the substituents, totally call the shots on where it reacts. Think of it like this: salicylamide is the main character in our little chemical drama, and these substituents are the supporting cast, each with their own personality, ready to nudge our hero in one direction or another. It's like a superhero movie, but instead of capes and laser eyes, we've got electron-pushing and ring-activating!
So, what's salicylamide all about? Imagine a little benzene ring, you know, that six-carbon circle that's everywhere in organic chemistry. Now, stick an amide group (think of it as a fancy nitrogen and oxygen combo) and a hydroxyl group (that's just an oxygen with a hydrogen attached) onto that ring. Bam! You've got salicylamide. It’s a pretty neat molecule on its own, but things get really interesting when we start messing with it.
Our star players, the substituents, are like little signposts attached to the benzene ring. They're not just hanging out; they're actively influencing where the next chemical party will happen. And trust me, these substituents have strong opinions! Some are like the super-enthusiastic friends who are always saying, "Go this way! It's so much fun over here!" while others are more like the cautious types, whispering, "Maybe not that direction, it looks a bit… intense."
Let's talk about the superstars, the electron-donating groups. These guys are the life of the party! They're like those friends who always bring the loudest music and the most delicious snacks, making everyone around them feel energized and ready to mingle. When you slap one of these electron-donating groups onto our salicylamide ring, it basically throws a big ol' party for any incoming guests (other molecules looking to react). These groups make the ring electron-rich, like a popular spot on a Saturday night. They make certain positions on the ring, particularly the spots ortho (next door) and para (directly opposite), incredibly attractive. It's like they're putting up giant neon signs that say, "ATTENTION, REACTION LOVERS! PRIME SPOTS AVAILABLE HERE!"
Think about a methyl group (that's just a carbon with a few hydrogens) or an alkoxy group (that's like an oxygen with a little carbon chain attached). These are our friendly neighborhood electron-donators. They're pushing electron density into the ring, making it more willing to jump into reactions. It’s like giving your salicylamide a shot of pure enthusiasm! Suddenly, the spots next to it and across from it are just begging to be explored.
But wait, there's a whole other crew in town: the electron-withdrawing groups. These guys are the chillers, the ones who might say, "Whoa there, let's pump the brakes a bit." Instead of inviting more electrons to the party, they're sort of… borrowing them. They pull electron density away from the ring, making it less excited about getting involved. They're like the strict chaperones who tell everyone to keep it down. This makes the ring less reactive overall.
Imagine a nitro group (that's a nitrogen and two oxygens, looking all serious) or a carbonyl group (that's a carbon double-bonded to an oxygen, very business-like). These are our electron-withdrawing wizards. They create a bit of a buzzkill for the ring, making it less keen on jumping into reactions. When these are around, the ring becomes a bit more reserved, like a library during study week. They can even direct incoming reactions to the meta position, which is the spot between the ortho and para positions. It's like they're saying, "Yeah, I'm not really into the main crowd. I prefer a quieter corner over there."
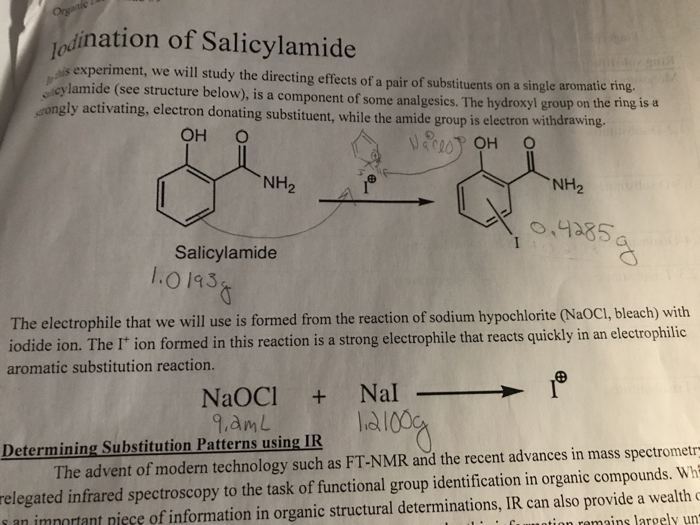
Now, what makes salicylamide so special is that it already has two handy groups attached: the hydroxyl and the amide. The hydroxyl group is a pretty good electron-donator. It’s like the host of the party who’s really good at making guests feel welcome and encouraging them to spread out and have a good time. It loves to activate those ortho and para positions.
The amide group is a bit more of a mixed bag. Depending on how you look at it and the conditions, it can sometimes lean towards being an electron-withdrawer, especially the nitrogen atom. It’s like a guest who’s generally friendly but has a few quirks. However, the hydroxyl group is usually the louder, more influential voice in the room when it comes to directing reactions. It’s the one making the big decisions!

So, when we add another substituent to our salicylamide party, we've got a whole committee deciding where the next chemical action will take place. If we add a strong electron-donator, like a methoxy group (which is like a methyl group attached to an oxygen), it’s going to team up with the existing hydroxyl group to create a super-activated ring. Those ortho and para spots are going to be practically vibrating with anticipation! It’s like two enthusiastic hosts high-fiving and saying, "This is going to be the best party ever!"
But if we add a strong electron-withdrawer, say a cyano group (that’s a carbon triple-bonded to a nitrogen, very sharp!), it’s going to try and dial down the enthusiasm. It'll be like, "Whoa, slow down, everyone!" In this case, the directing effects can get a little more complicated, but generally, the existing groups still have their say, and the new guy will influence things too. It’s like having a debate at the party about the best music genre.
Understanding these directing effects is like having a secret map to where chemical reactions will occur. It’s not just random chaos; it’s a dance choreographed by the electronic personalities of these substituents. It’s what makes chemistry so fascinating – predicting the outcome, seeing how these tiny molecular interactions lead to big changes. So next time you hear about salicylamide, remember it’s not just a molecule; it’s a social scene, and the substituents are the ultimate influencers, dictating the flow of the chemical conversation! It’s pure molecular magic, and we get to peek behind the curtain!
