Classifying Chemical Reactions Analyzing And Predicting Products
Hey there! So, you're diving into the wild world of chemistry, huh? It's like a giant puzzle, and today, we're going to tackle a super cool piece of it: classifying chemical reactions. Think of it as learning to sort your Lego bricks so you can actually build something awesome, instead of just staring at a giant pile. It’s way less messy than actual Legos, by the way. We're talking about understanding what's happening when stuff changes, and that, my friends, is pretty darn neat.
Imagine you've got two things hanging out, maybe a couple of energetic molecules just chilling. Then, BAM! Something happens. They interact, they swap partners, they might even explode (okay, maybe not explode every time, but you get the idea). Chemical reactions are basically nature's way of saying, "Let's mix things up!" and seeing what new goodies come out. It’s like a molecular makeover!
And the best part? Once you get the hang of classifying them, you can start to predict what's going to happen. It's like having a crystal ball for chemistry. No more guessing games, just informed predictions. How cool is that? You’ll be the mad scientist everyone asks for advice, even if you're just making toast.
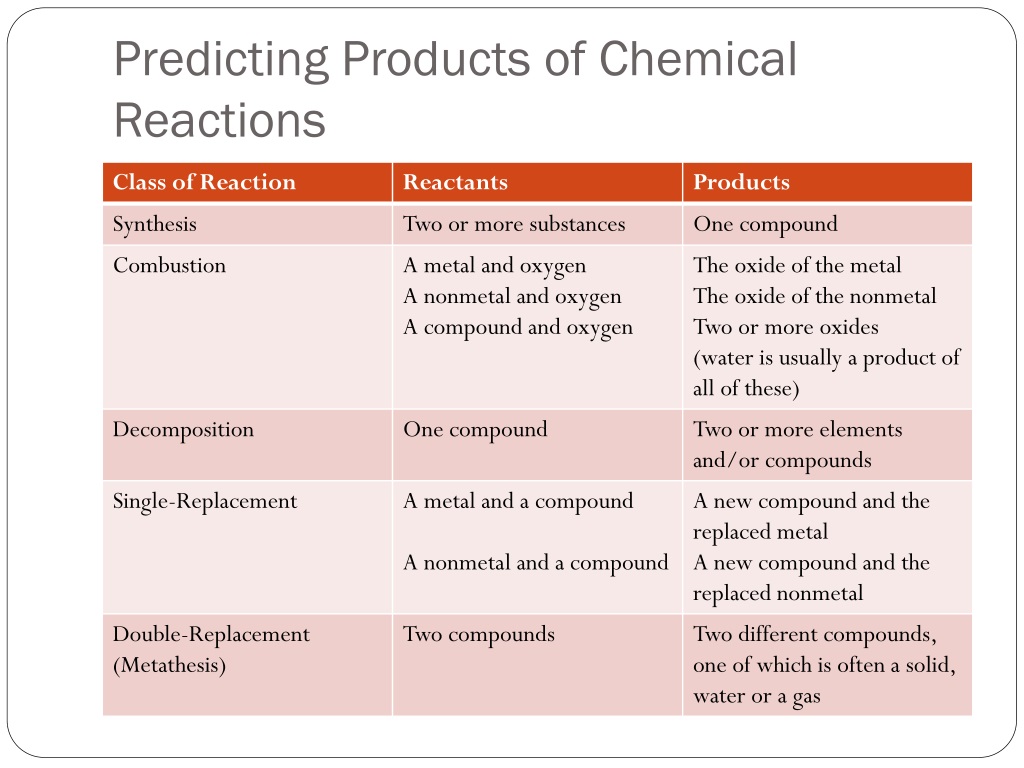
The Big Categories: Your Reaction Sorting Hat
So, how do we sort these molecular shindigs? Chemists, bless their organized little hearts, came up with a few main categories. It’s not like there’s one universal rule, but these are the heavy hitters, the ones you’ll see over and over. Think of them as your main chapters in the giant chemistry textbook of life.
These classifications aren't just for fun, you know. They help us understand the mechanism of the reaction, which is basically the step-by-step process. It’s like figuring out how a magic trick is done – once you know the steps, you can kind of anticipate the outcome, or even do the trick yourself. Pretty handy, right?
Synthesis: The "Let's Make Something New!" Party
First up, we've got synthesis reactions. These are the builders, the creators, the ones where two or more things decide to join forces and make something bigger and usually more complex. It’s like two single molecules going on a date and deciding to get married and start a family. Aww!
The general idea is pretty straightforward: A + B → AB. See? Simple. You start with separate ingredients, and you end up with a brand new compound. Think of it like baking a cake. You start with flour, eggs, sugar, and when you bake them together, you get a delicious cake. The cake is the new compound! Your kitchen might smell amazing, which is a definite perk.
A classic example is when hydrogen gas (H₂) and oxygen gas (O₂) combine to form water (H₂O). So, 2H₂ + O₂ → 2H₂O. It seems so simple, but this is the reaction that makes… well, everything wet! And it’s vital for life. Who knew that a little bit of combining could be so important? You can’t really make water out of thin air, you need those building blocks to come together.

Or, how about when metals react with nonmetals? Like sodium (Na) and chlorine (Cl) making sodium chloride (NaCl) – that’s table salt! So, 2Na + Cl₂ → 2NaCl. Now you have something you can sprinkle on your fries. Pretty neat transformation, wouldn't you say? From two reactive elements to a delicious (in moderation!) condiment.
The key thing to remember here is that you’re building something. You’re not breaking apart existing things, you’re putting them together. It’s all about addition. If you see a reaction where multiple simpler substances are combining to form a single, more complex substance, chances are it's a synthesis. It's the "more is more" approach of the chemical world.
Decomposition: The "Let's Break This Apart" Process
On the flip side, we have decomposition reactions. These are the exact opposite of synthesis. Instead of building, we’re breaking. Think of it like a sad little molecule that can't handle the pressure anymore and decides to split up. It's the chemical equivalent of a dramatic breakup. So dramatic!
The general form is AB → A + B. One thing goes in, and two or more things come out. It’s like taking that cake you baked and trying to un-bake it. Good luck with that! Usually, these reactions need a little push, like heat or electricity, to get them going. Nature doesn't usually just spontaneously break things apart without a good reason (or some energy input).
A common example is the decomposition of hydrogen peroxide (H₂O₂). You know that stuff you use to clean cuts? When it breaks down, it forms water (H₂O) and oxygen gas (O₂). So, 2H₂O₂ → 2H₂O + O₂. That's why you see bubbles when you pour it on a wound – that's the oxygen gas escaping! It's like a little chemical sigh of relief.
Another one is the heating of calcium carbonate (CaCO₃), which is limestone. When you heat it up, it breaks down into calcium oxide (CaO) and carbon dioxide gas (CO₂). CaCO₃ → CaO + CO₂. This is actually how we get lime for building and agriculture. So, that boring rock can be transformed into useful stuff!
So, if you see a reaction where a single compound is breaking down into two or more simpler substances, you’re probably looking at a decomposition reaction. It's all about reduction, about taking something apart. It’s the "less is more" approach, but in a way that often yields useful smaller components.
Single Replacement: The "Swap Partners" Shuffle
Now, things get a little more interesting with single replacement reactions. This is where one element decides to kick another element out of a compound and take its place. It’s like a dance where one person cuts in and steals their partner. Awkward!
The general form looks something like A + BC → AC + B. Element A comes along, sees compound BC, and thinks, "You know what? I'm better than B. I'm going to take BC's spot." And, if A is reactive enough, it does! Element B is then left all by its lonesome.
A classic example is when a metal reacts with an acid. For instance, zinc metal (Zn) reacting with hydrochloric acid (HCl). Zinc is more reactive than hydrogen, so it kicks the hydrogen out of the HCl and forms zinc chloride (ZnCl₂), with hydrogen gas (H₂) being released. So, Zn + 2HCl → ZnCl₂ + H₂. You can actually see the bubbles of hydrogen gas forming!
Or, a more reactive metal can replace a less reactive metal in a solution. Like if you put a piece of copper (Cu) into a solution of silver nitrate (AgNO₃). Copper is more reactive than silver, so it kicks silver out and forms copper(II) nitrate (Cu(NO₃)₂), leaving silver metal (Ag) to precipitate out. Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag. Suddenly, your clear solution might get a little cloudy as silver crystals form. It’s like a little metallic art project.
The key here is the idea of activity series. Elements have a ranking of how reactive they are. A more reactive element can replace a less reactive one, but not the other way around. It's like a chemical pecking order. If you don't know the activity series, predicting these can be tricky. But once you do, it’s like unlocking a secret code.

Double Replacement: The "Musical Chairs" of Molecules
Finally, we have double replacement reactions, also known as double displacement. This is like a game of musical chairs for ions. The positive ion from one compound switches places with the positive ion from another compound. It’s a real partner swap! Chaos, but organized chaos!
The general form is AB + CD → AD + CB. You start with two compounds, and the cations (positive ions) and anions (negative ions) switch partners. It's like AB and CD are dating, and then A decides to date D, and C decides to date B. Everyone finds a new partner!
These reactions often happen in aqueous solutions (solutions where water is the solvent). And the driving force for these reactions is usually the formation of something that removes ions from the solution. This could be a precipitate (an insoluble solid), a gas, or water. If you don’t form one of these, the reaction usually doesn’t go anywhere. It’s like the musical chairs music stops, but everyone is still standing!
A classic example is the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl). Both are soluble in water. When you mix them, the silver ion (Ag⁺) from silver nitrate pairs up with the chloride ion (Cl⁻) from sodium chloride to form insoluble silver chloride (AgCl), which precipitates out as a white solid. The sodium ion (Na⁺) from sodium chloride pairs up with the nitrate ion (NO₃⁻) from silver nitrate to form soluble sodium nitrate (NaNO₃), which stays dissolved. So, AgNO₃ + NaCl → AgCl(s) + NaNO₃. You see a cloudy solid form!
Another example is when an acid reacts with a base. This is called a neutralization reaction, and it's a type of double replacement. For instance, hydrochloric acid (HCl) reacting with sodium hydroxide (NaOH). The H⁺ from HCl and the OH⁻ from NaOH combine to form water (H₂O). The Na⁺ from NaOH and the Cl⁻ from HCl combine to form sodium chloride (NaCl). So, HCl + NaOH → H₂O + NaCl. It’s a classic acid-base reaction, and it’s pretty darn important for things like regulating the pH of your blood!
The key to predicting double replacement reactions is to know which compounds are soluble and which are insoluble in water. You’ll learn about solubility rules, and those are your best friends here. If mixing two solutions results in the formation of an insoluble solid, a gas, or water, then you've likely got a double replacement reaction happening.
Putting It All Together: The Art of Prediction
So, we’ve covered the big four: synthesis, decomposition, single replacement, and double replacement. Now, how do we use this to predict what’s going to happen? It’s like being a detective, looking for clues!
First, you need to identify the reactants. What are the starting materials? Are they elements? Compounds? Are they dissolved in water?
Then, you look at the patterns. Does it look like multiple things are coming together to form one big thing? (Synthesis!) Does one big thing seem to be breaking apart into smaller pieces? (Decomposition!) Is there a lone element looking to join a compound, or to kick something out? (Single Replacement!) Are you mixing two ionic compounds and looking for a partner swap? (Double Replacement!)
You'll also need to consider things like heat, light, or electricity. These can be energy inputs that drive reactions, especially decomposition. And don’t forget about reactivity. For single replacement, you must know your activity series. For double replacement, those solubility rules are non-negotiable. They’re like your cheat sheet for knowing if a reaction will actually do anything.
It’s not always going to be perfectly clear-cut, of course. Chemistry can be sneaky! Sometimes reactions can be more complex, or they might fit into more than one category. But these basic classifications are your foundation. Master these, and you’ll be well on your way to understanding and predicting a huge chunk of the chemical world.
Think of it as building a vocabulary. Once you know the words for different types of actions, you can start to construct more complex sentences and understand longer stories. And in chemistry, the "stories" are how the universe itself works! Pretty mind-blowing when you stop and think about it, right? So go forth, classify those reactions, and start predicting like a pro. Your journey into the molecular mysteries has just begun!
