Classify The Transformation As A Reduction Oxidation Or Neither
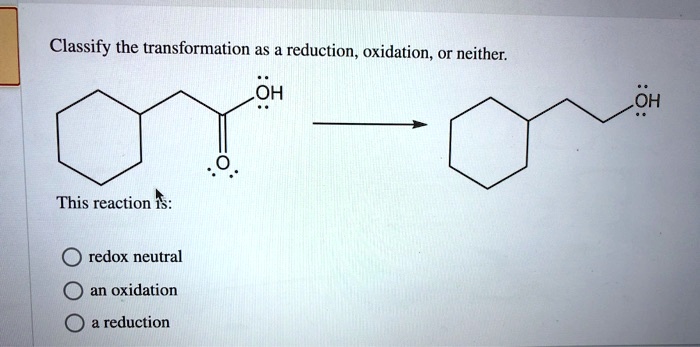
Ever looked at a banana and thought, "Wow, that's really going through some stuff"? Or perhaps you've witnessed a car rust into a magnificent shade of orange and pondered the mysteries of chemistry? Well, my friends, you've been on the front lines of chemical transformations without even knowing it! Today, we're going to dive into the sometimes-confusing, often-hilarious world of classifying these changes. Think of it like sorting your socks: some are just socks, some have lost their elastic, and some… well, some have mysteriously vanished. We're talking about Reductions, Oxidations, and the wonderfully mundane category of Neither.
Let's start with the rockstar of the show, the one that gets all the dramatic flair: Oxidation. You know how your shiny new bike gets that lovely rusty patina after a few rainy days? That, my friends, is oxidation in action! It’s like when you leave your favorite snack out on the counter too long, and it starts to look a little… off. It's not necessarily a bad thing, but it's definitely a change. Think of it as losing something precious. In chemistry terms, oxidation is all about an atom or molecule losing electrons. Imagine your friend is hoarding all the best cookies, and then suddenly, poof! They give one away. That’s oxidation. They’ve been oxidized, losing a cookie (an electron).
We often associate oxidation with oxygen, and for good reason! Oxygen is a real electron hog. It’s like that one friend at a party who can’t help but grab all the chips. When oxygen gets involved, it’s usually eager to snatch electrons away from other things. So, when iron meets oxygen, it surrenders its electrons, becoming iron oxide – the fancy scientific term for rust. It’s not a breakup, just a fundamental change in who’s holding what.
Think about when you cut an apple and leave it out. That browning? Yep, oxidation. The apple is saying goodbye to some electrons and hello to a different chemical identity. It’s like your phone battery slowly draining – it's losing its "charge," or in chemistry, its electrons. It’s a gradual process, sometimes subtle, sometimes glaringly obvious. And just like that browning apple, it’s a transformation. It’s not going back to being a crisp, white slice of apple without some serious effort (or a time machine!).
Now, let’s talk about the quiet hero, the yin to oxidation's yang: Reduction. If oxidation is about losing electrons, reduction is the magnanimous act of gaining them. It's the opposite of oxidation, like finding a perfectly matched sock after a laundry marathon. Remember our cookie-hoarding friend? Reduction is when someone else generously gives them a cookie. That friend, having just received a cookie (an electron), has been reduced. It's all about gaining.
In a chemical reaction, when one substance is oxidized (loses electrons), another substance must be reduced (gains those electrons). They’re like a cosmic exchange program. You can't lose something if nobody's there to pick it up! So, if iron is losing electrons to oxygen, oxygen is happily gobbling them up. Oxygen, in this case, is being reduced. It’s a team sport, a chemical ballet.
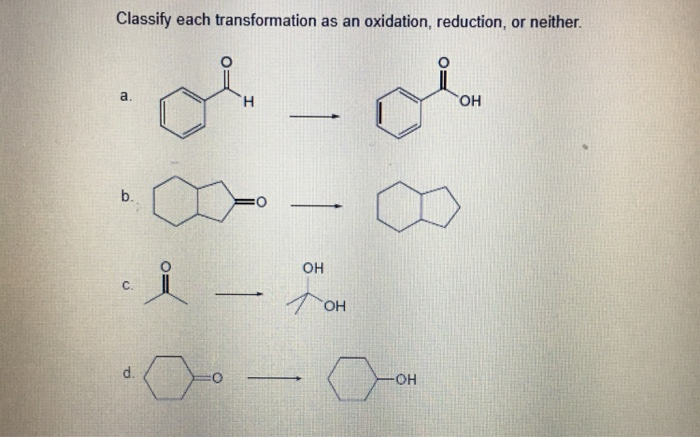
Think about your metabolism. Your body is constantly performing these little electron transfers to keep you going. When you eat food, your body "breaks it down," and in that process, electrons are exchanged. Some molecules get oxidized, and others get reduced. It’s how you get energy! It’s not always a dramatic explosion; sometimes it’s a quiet, steady hum of chemical activity keeping your lights on. When you take a deep breath, the oxygen you inhale is crucial. It’s often the electron acceptor, the one ready to receive those electrons and help keep your internal processes humming. Without reduction happening, life as we know it would be… well, a lot less energetic.
Now, here's where things can get a little tricky. Not every chemical change involves a give-and-take of electrons. Sometimes, things just… change. This is where we get our wonderfully non-committal category: Neither. These are the transformations that don't fit the electron-transfer mold. They're the chemical equivalent of changing your shirt because you spilled something, not because you lost or gained a vital organ.
Imagine you're boiling water. The water molecules are still H₂O, and the atoms within them haven't swapped any electrons with each other. They're just moving faster, getting more energetic, and that's a transformation! The water has gone from a placid pool to a bubbling frenzy. It’s a physical change, not a redox reaction. It’s like rearranging the furniture in your house. The furniture is still the same, just in a different spot or orientation. No electrons were exchanged in the process of moving that couch.
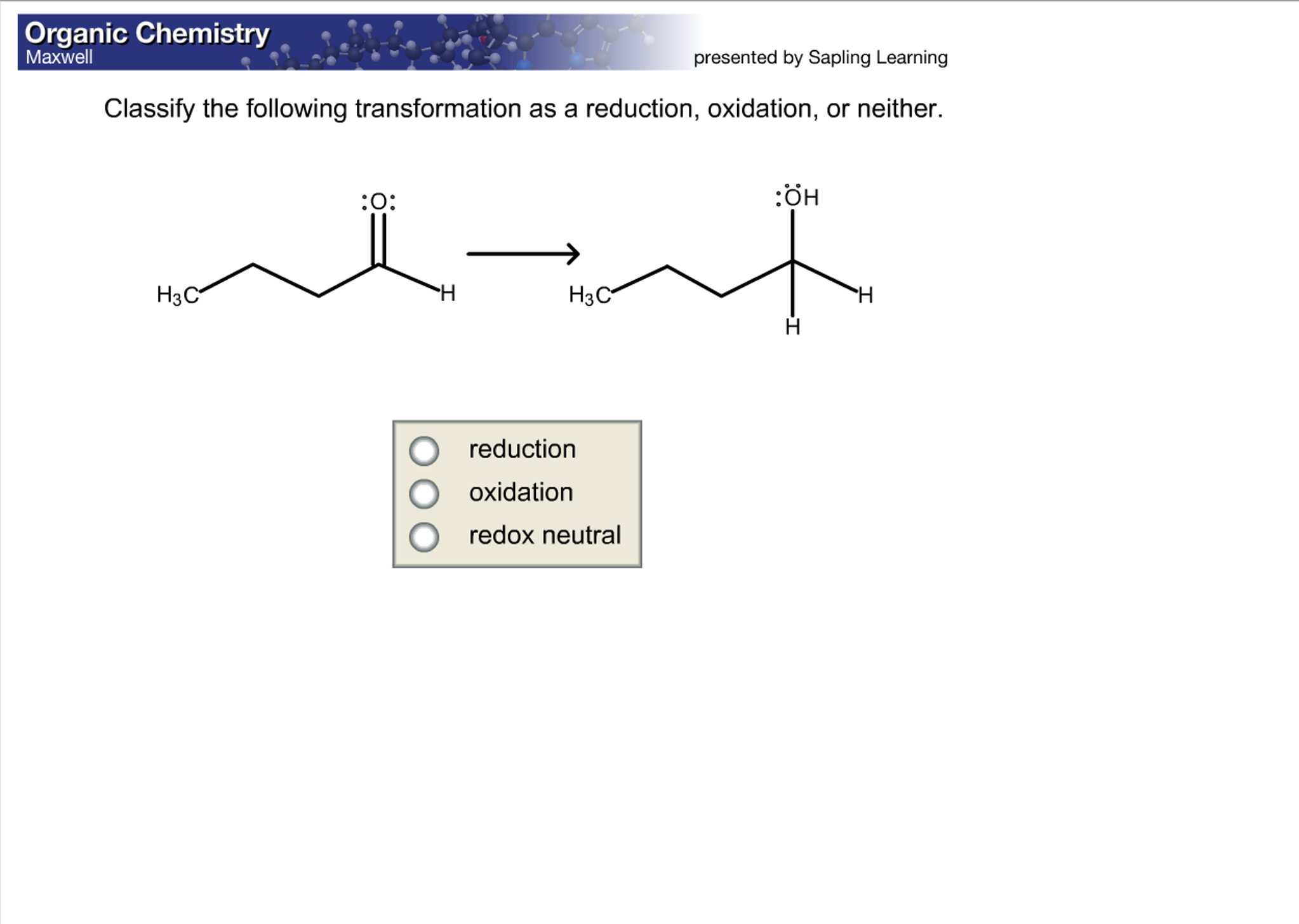
Or consider dissolving sugar in water. The sugar molecules are still sugar molecules, and the water molecules are still water molecules. They're just mixed up in a sweet, sugary solution. No electron trading is happening. It’s like adding sprinkles to your ice cream. The ice cream is still ice cream, and the sprinkles are still sprinkles. They’re just enjoying each other’s company. This "neither" category is where the majority of everyday physical changes reside. It’s the mundane magic of mixing, melting, freezing, and dissolving.
So, how do we tell these apart in the wild? It’s like being a detective for molecules! The biggest clue is to look for changes in oxidation states. Don't let that fancy term scare you. Think of oxidation states like a person's "score" for how much they've either gained or lost electrons relative to a neutral state. If a substance’s oxidation state changes during a reaction, you're probably dealing with an oxidation or a reduction. If those scores stay the same for all the atoms involved, then it’s likely a "neither" situation.
Let's get a little more concrete. We often use the concept of "loss of electrons is oxidation, gain of electrons is reduction" – remember OIL RIG? Oxidation Is Loss, Reduction Is Gain. It’s a handy little mnemonic that’s been saving many a confused student (and adult!) from chemical despair. Think of it as your secret code word for cracking the chemical case.
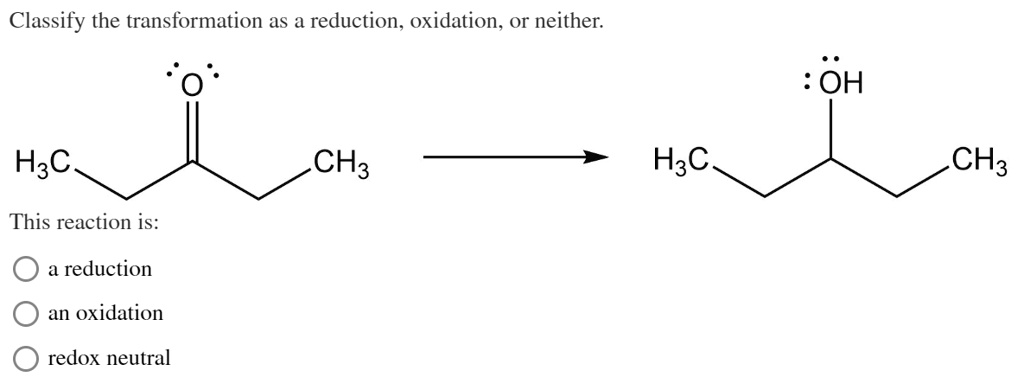
Consider the combustion of methane (CH₄), the main component of natural gas. When methane burns, it reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). Let's break it down like we're dissecting a mystery novel. In methane, the carbon atom is in a state where it's somewhat "electron-rich" (oxidation state of -4). When it forms CO₂, the carbon is now much more "electron-poor" (oxidation state of +4). It lost electrons! So, the carbon in methane has been oxidized.
Where did those electrons go? To the oxygen! In O₂, oxygen atoms are pretty neutral. But in CO₂ and H₂O, they've effectively gained electrons (their oxidation state goes from 0 to -2 in both products). So, the oxygen has been reduced. See? One goes up, one goes down, like a chemical seesaw. This is a classic redox reaction.
Now, let's look at something simpler. What about the electrolysis of water? We're splitting water (H₂O) into hydrogen gas (H₂) and oxygen gas (O₂). In water, the oxygen has an oxidation state of -2. In O₂, it has an oxidation state of 0. It lost electrons (went from -2 to 0). So, oxygen is oxidized. The hydrogen in water has an oxidation state of +1. In H₂, it has an oxidation state of 0. It gained electrons (went from +1 to 0). So, hydrogen is reduced. Another redox reaction, albeit one that requires electricity to force it to happen!
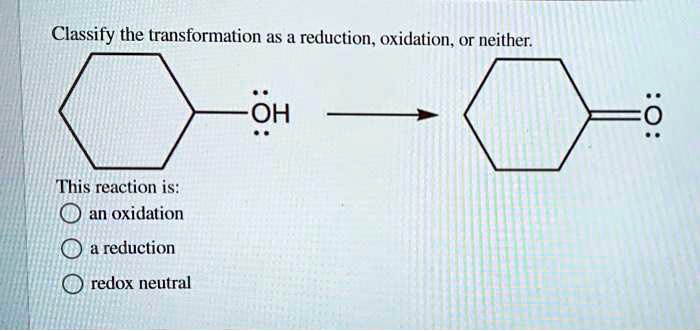
What about something like the neutralization of an acid and a base? For example, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H₂O). Let's check our oxidation states. In HCl, H is +1 and Cl is -1. In NaOH, Na is +1, O is -2, and H is +1. In NaCl, Na is +1 and Cl is -1. In H₂O, H is +1 and O is -2. Notice anything? The oxidation states of all the atoms remain exactly the same before and after the reaction! Sodium is always +1, chlorine is always -1, hydrogen is always +1 (except when it's H₂), and oxygen is always -2 (except when it's O₂). Since there's no change in electron distribution (no gain or loss of electrons), this reaction is classified as neither oxidation nor reduction. It's a classic acid-base reaction, a simple mixing and rearranging, but not an electron rodeo.
Think about cooking. When you bake a cake, you're not typically performing a redox reaction. You're changing the physical structure of the ingredients, denaturing proteins, and creating new compounds through heat and mixing. The flour, eggs, and sugar are undergoing transformations, but it's more about rearranging molecules and changing their physical properties than a dramatic electron exchange. Unless you're grilling some steak, where the browning is a form of oxidation (the Maillard reaction, which is a complex series of reactions, but a significant part of it involves oxidation), most baking is in the "neither" category.
The key takeaway is to be a bit of a detective. Look for the clues! Is something reacting with oxygen (often a giveaway for oxidation)? Is there a clear gain or loss of electrons involved? Or are things just getting mixed, heated, or cooled, with their fundamental electron counts staying the same? Don't be intimidated by the jargon. At its heart, it’s all about understanding how atoms and molecules interact, how they share, steal, or keep their electrons. It’s the unseen drama playing out all around us, from the rust on your gate to the energy that powers your life. So next time you see something change, take a moment to ponder: is it a dramatic electron heist, a generous electron gift, or just a simple rearrangement of the molecular furniture? The answer is usually just a chemical observation away!
