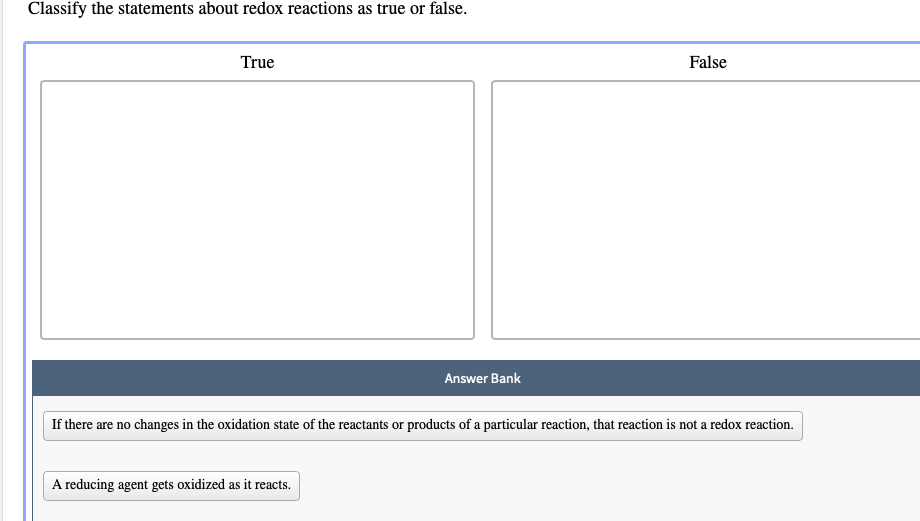
Classify The Statements About Redox Reactions As True Or False.
Ever feel like you're playing a guessing game with science? Well, get ready for a little quiz about redox reactions! These are the unsung heroes of all sorts of everyday magic.
The Great Redox Riddle
Think of redox reactions as a cosmic dance of electrons. Sometimes they're being passed around, sometimes they're being hoarded. It's a whole lot of electron drama!
Let's see if you can spot the truth from the tall tales in this electron-y saga. No need to pack your lab coat; we're keeping it light and breezy!
"Redox reactions: where electrons go to party... or sometimes get taken hostage."
Statement 1: Rusting iron is a redox reaction.
Imagine a piece of your favorite metal sculpture, left out in the rain. It slowly gets that reddish-brown coating. That's rust, and it's a classic example of a redox reaction at play.
The iron is basically giving away some electrons to the oxygen in the air. It's like the iron is saying, "Here, oxygen, you can have these!" and the oxygen is happily accepting. This electron exchange is the heart of rusting.
So, is this statement true or false? Think about the electron transfer.
Statement 2: When you breathe, a redox reaction is happening in your body.
Every single breath you take is a marvel of chemistry! Your body is constantly doing these amazing electron exchanges to give you energy. It's like a tiny, efficient power plant inside you.
When you inhale oxygen, it's not just filling your lungs. It's starting a cascade of reactions that help break down food molecules for fuel. This process involves electrons being passed along, much like a tiny baton in a cellular relay race.
This one is a pretty big deal for staying alive. So, is it true or false?

Statement 3: Burning wood is not a redox reaction.
Picture a cozy campfire on a chilly evening. The flames dance, the wood turns to ash, and you feel all warm and fuzzy. There's a lot going on chemically there!
Burning, or combustion, is a very rapid redox reaction. The fuel (wood) loses electrons, and the oxygen from the air gains them, releasing a huge amount of energy as heat and light. It's a rather enthusiastic electron exchange!
So, is the idea that burning wood isn't a redox reaction true or false?
Statement 4: Adding sugar to your tea is a redox reaction.
Ah, the simple pleasure of sweetening your tea! You stir in that white granulated goodness, and it dissolves. Does this involve a wild electron chase?
While sugar is a fascinating molecule with lots of electrons, simply dissolving it in water doesn't typically involve a significant transfer of electrons between sugar and water molecules in a way that we define as a redox reaction. It's more about breaking bonds and forming new interactions.
Think about what actually happens to the electrons. Is this statement true or false?

Statement 5: Batteries work because of redox reactions.
Your phone, your remote control, your car – they all rely on the magic of batteries! And what makes those batteries tick? You guessed it: redox reactions.
Inside a battery, there are two different materials that have a strong desire to gain or lose electrons. One material readily gives up electrons (gets oxidized), and the other eagerly accepts them (gets reduced). This flow of electrons through an external circuit is what we call electricity.
It's a beautifully controlled electron flow, powering our modern lives. So, is this statement about batteries true or false?
Statement 6: Photosynthesis is a redox reaction.
Plants are nature's little alchemists, turning sunlight, water, and carbon dioxide into food and oxygen. This incredible process is called photosynthesis, and it's a redox masterpiece.
In photosynthesis, water molecules are split, releasing electrons. These electrons are then used to convert carbon dioxide into sugars. Meanwhile, oxygen is released as a byproduct.
It's a complex but elegant electron juggling act. Is photosynthesis a redox reaction, making this statement true or false?
Statement 7: Cooking an egg involves a redox reaction.
The transformation of a clear, gooey liquid into a solid, delicious egg is quite something. But does it involve electrons swapping sides?
While heat does cause chemical changes in an egg, primarily involving the denaturation and re-folding of proteins, it's not typically classified as a redox reaction. The main changes are structural and bond rearrangements, not a significant net transfer of electrons between distinct chemical species in the way redox reactions typically do.
So, when you cook that perfect omelet, are you witnessing a redox event? Is this statement true or false?
Statement 8: The process of digestion is a redox reaction.
Your stomach is a busy place! It's breaking down the food you eat into tiny pieces your body can use. This complex process involves many chemical reactions.
Digestion involves a lot of breaking chemical bonds and rearranging molecules. Some of these reactions can be redox reactions, especially when your body is extracting energy from food. Think about how your body "burns" fuel from your meals.
Given that our bodies are constantly working to get energy from food, is digestion a redox reaction, making this statement true or false?
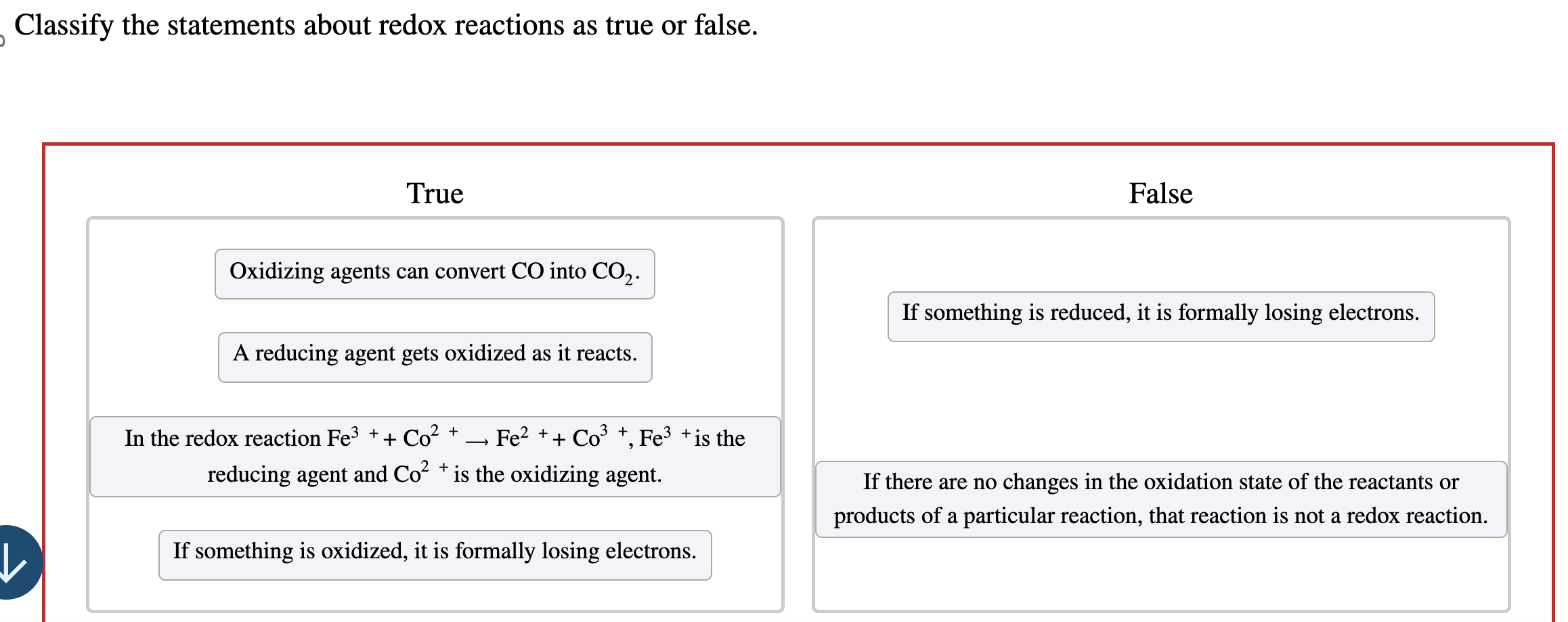
Statement 9: Antacids neutralize stomach acid through redox reactions.
When your tummy feels a bit too acidic, you might reach for an antacid. These little helpers calm down that burning sensation. But how do they do it?
Antacids work by a process called neutralization, which is an acid-base reaction. They contain bases that react with the acid in your stomach to form salt and water, reducing the acidity. This is generally not a redox reaction, as there isn't a significant transfer of electrons.
So, are antacids performing electron acrobatics? Is this statement true or false?
Statement 10: Exploding fireworks rely on redox reactions.
The dazzling light shows and booming sounds of fireworks are pure spectacle. They're designed to impress and excite! And the science behind them is pretty explosive.
Fireworks are essentially controlled explosions, and explosions are almost always a sign of rapid redox reactions. The chemicals in the fireworks are designed to react vigorously with each other, involving rapid oxidation and reduction, releasing massive amounts of energy, light, and sound in a spectacular display.
It's a dramatic demonstration of electrons on the move. Is this statement about fireworks true or false?
So, how did you do? Hopefully, you found this little electron adventure fun and illuminating!
