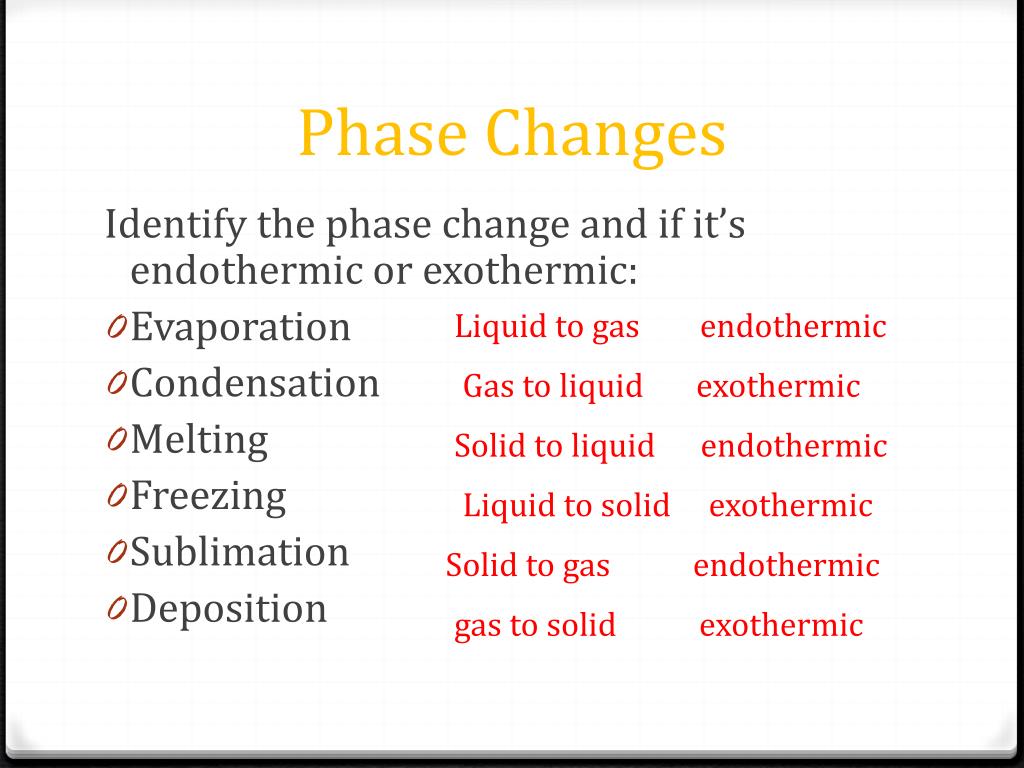
Classify The Following Phase Changes As Endothermic Or Exothermic
Hey there, science enthusiasts and fellow humans who've ever stared blankly at a melting ice cube or wondered why your breath turns into a little cloud on a chilly morning! We're diving headfirst into the wonderfully weird world of phase changes, and let me tell you, it's not as complicated as it sounds. Think of it like your mood swings, but for molecules. Sometimes they need a little extra oomph (energy!) to get jazzed up and switch things around, and sometimes they're so chill they just let go of that energy like it's last season's fashion.
We're going to break down whether these changes are endothermic (think of it as needing a hug – they’re absorbing energy) or exothermic (they’re letting go of energy, like a sigh of relief after a long day). No need for lab coats or beakers here, just your trusty brain and maybe a comfy chair. So, grab a cuppa, settle in, and let’s get our science on, the easy-going way!
The Big Kahuna: What Exactly IS a Phase Change?
Alright, let’s get down to basics. A phase change is basically when a substance decides to ditch its current state of matter for a new one. You know, like water. It can be a solid (ice), a liquid (the stuff you drink or use to make tea), or a gas (steam, that steamy cloud you see when you boil water, or even the invisible stuff all around us).
Imagine your favorite celebrity. They can be on the red carpet looking all glamorous (solid), chilling backstage with their entourage (liquid), or totally blasting their new album on tour, reaching fans everywhere (gas). It's the same celebrity, just in different "phases" of their career, or life, or whatever. See? Not so scary!
These shifts happen when we add or remove energy, usually in the form of heat. It’s like life’s little nudges and shoves that get things moving. Sometimes a little poke is all it takes, other times you need a full-on motivational speaker.
Endothermic: The Energy Grabbers
Let’s talk about the endothermic folks. These are the substances that are a bit like your perpetually hungry teenager. They’re always looking for more energy, constantly absorbing it to make their big transition. Think of it as them needing a big ol' hug of energy to get going.
When a substance undergoes an endothermic phase change, it’s taking heat from its surroundings. This means the surroundings might feel a bit cooler, like when you use an instant cold pack. That pack is busy absorbing heat from your sore ankle to make itself cold, and your ankle feels better. Win-win!
Melting: The Great Escape of Ice
This is a classic! When ice melts into water, it's an endothermic process. That solid block of ice needs to absorb energy from its surroundings to break free from its rigid structure. It’s like the ice cubes in your drink are saying, "Ugh, this is so stiff! I need to loosen up!" And poof, they melt.
Think about it: if you leave an ice cube out on a warm summer day, it doesn’t spontaneously freeze more. It melts. It’s actively grabbing that ambient heat to become liquid. That’s why your drink gets colder when you add ice – the ice is stealing the heat from the drink to melt. It’s a heat heist, and the ice is the mastermind!
Imagine you’re stuck in a really boring, rigid job. You’re like a solid, perfectly structured… well, something. Then, suddenly, you get offered an amazing vacation with unlimited snacks. That’s the energy! You absorb that exciting energy, break free from your rigid job structure, and suddenly you’re a relaxed, flowing liquid, ready to explore. That’s melting, folks. Energy in, structure out.
Vaporization (Boiling and Evaporation): Getting a Gaseous Glow-Up
Next up, we have vaporization. This is when a liquid turns into a gas. Whether it's a dramatic boil or a subtle evaporation, it's endothermic. The molecules in the liquid need a serious energy boost to break those intermolecular bonds and float off into the gaseous realm.
Boiling water for pasta? That’s endothermic. The stove is pumping energy into the water, and the water is soaking it all up like a sponge before it decides to become steam. You can feel the heat from the stove, but the water is the one doing the heavy lifting, energetically speaking. It’s like the water molecules are saying, "We need to escape this watery prison! More heat, please!"
Evaporation is the quieter cousin. Think about your wet clothes drying on the line. They’re not exactly boiling, but they are turning into water vapor. Where does that energy come from? The air around them, the sun, anything that’s a bit warmer. The water molecules are stealing that energy to make their escape. It’s a stealthier heat heist.
Here’s a funny one: remember that time you spilled a bit of juice on the counter, and then you forgot about it? A few hours later, poof, it’s gone! Not magically cleaned up, but evaporated. That juice worked hard, absorbing ambient energy to break free and become invisible. It’s like a tiny, liquid escape artist.
So, if you’ve ever felt a cool breeze on your skin after swimming, that’s evaporation at work. The water on your skin is evaporating, and to do that, it’s stealing heat from your body. Makes you shiver, doesn’t it? All because those water molecules are power-hungry!
Sublimation: The Ghostly Transformation
This one is a bit more niche, but super cool. Sublimation is when a solid turns directly into a gas, skipping the liquid phase altogether. Think dry ice (solid carbon dioxide) turning into carbon dioxide gas. It doesn’t melt into a puddle; it just… fizzes away.

This is also an endothermic process. The solid needs to absorb a lot of energy to make that jump. It’s like deciding to skip your awkward teenage years and go straight to being a cool adult. You need some serious energy and a bold move to do that!
Ever seen those spooky fog effects at parties or Halloween events? That's dry ice sublimating. It’s absorbing heat from the air and creating that cool, wispy fog. It’s pure energy absorption for a dramatic effect. These molecules are basically saying, "Why bother with liquid? Let's go straight to the ethereal gas phase!"
Exothermic: The Energy Shedders
Now, let’s flip the coin. We have the exothermic phase changes. These are the substances that are a bit like a grumpy teenager who’s finally gotten their allowance. They’ve got extra energy and they’re just itching to get rid of it. They’re releasing heat into their surroundings.
When an exothermic phase change happens, the surroundings actually get warmer. It’s like a group project where one person does all the work and then just slumps back, radiating relief (and maybe a little bit of heat). These processes are the universe’s way of saying, "Okay, that was a lot of effort, let’s chill out and give some energy back."
Freezing: The Solidifying Hug
When water freezes into ice, it's an exothermic process. To become that rigid solid, the water molecules have to slow down and get closer. As they do, they release energy. It’s like they’re settling into a cozy group hug, and that hug releases warmth.
Think about building an ice rink. You need to remove heat from the water. Where does that heat go? It gets released into the surrounding air. That’s why in very cold environments, things can feel even colder when they’re freezing – the freezing process is actually adding a tiny bit of heat to the surroundings, but the overall process is about removing heat to achieve the solid state.

Imagine a bunch of hyperactive kids at a party. They're all over the place, buzzing with energy. Then, suddenly, it’s bedtime. They’re told to calm down, get into their pajamas, and snuggle into bed. As they settle down, they release that pent-up energy. That’s like freezing. Molecules calming down, getting organized, and letting go of excess energy.
Have you ever noticed that when it gets really, really cold, sometimes water pipes can freeze and burst? That’s because as water freezes, it expands. But more importantly, the process of freezing itself is exothermic. While it’s releasing a bit of heat, the overall cooling needed to initiate freezing is what causes the problem. But the release of energy during the phase change is key here.
Condensation: The Foggy Embrace
Condensation is the opposite of vaporization. It's when water vapor in the air turns back into liquid water. Think of the water droplets that form on the outside of a cold glass on a humid day, or the fog that appears when you breathe out on a cold day. This is an exothermic process.
The gas molecules have too much energy to stay together as a gas. They need to slow down and form bonds to become a liquid. As they do, they release energy. It’s like a group of shy introverts finally deciding to form a small, comfortable circle instead of milling around a huge room. That settling down releases a bit of nervous energy.
Ever seen dew on the grass in the morning? That’s condensation. The water vapor in the air cooled down, lost energy, and turned into liquid dew drops. The air itself might feel slightly warmer as this happens, or at least less humid. It's the gas molecules giving up their free-spirited ways for the more structured life of a liquid.
And that steamy mirror after a hot shower? That’s condensation too. The warm water vapor from the shower hits the cooler mirror, loses energy, and turns back into tiny liquid water droplets. The mirror might feel a tiny bit warmer where the steam hits it. It’s like the water vapor is saying, "Whew, that was exhausting being all floaty. I need a break, and here’s some warmth for you as a thank you!"
Deposition: The Direct Solidification
Deposition is the opposite of sublimation. It’s when a gas turns directly into a solid, skipping the liquid phase. Frost forming on a windowpane on a very cold, dry night is a classic example. This is also an exothermic process.

The gas molecules are in a high-energy state and need to lose a lot of energy to become a solid. It’s like a rockstar deciding to immediately go from a massive concert (gas) to a quiet monastic life (solid) without any downtime. That’s a big energy shift!
Think about those delicate frost patterns you sometimes see on windows. That’s water vapor from the air directly turning into ice crystals. The gas molecules are giving up their energy to arrange themselves into a solid structure. They’re essentially saying, "Too much energy! Let's just freeze into a beautiful pattern right now!"
Putting It All Together: The Grand Molecular Dance
So, there you have it! Phase changes are just the universe’s way of shuffling molecules around, and whether they’re grabbing energy (endothermic) or giving it back (exothermic) depends on the direction of the dance.
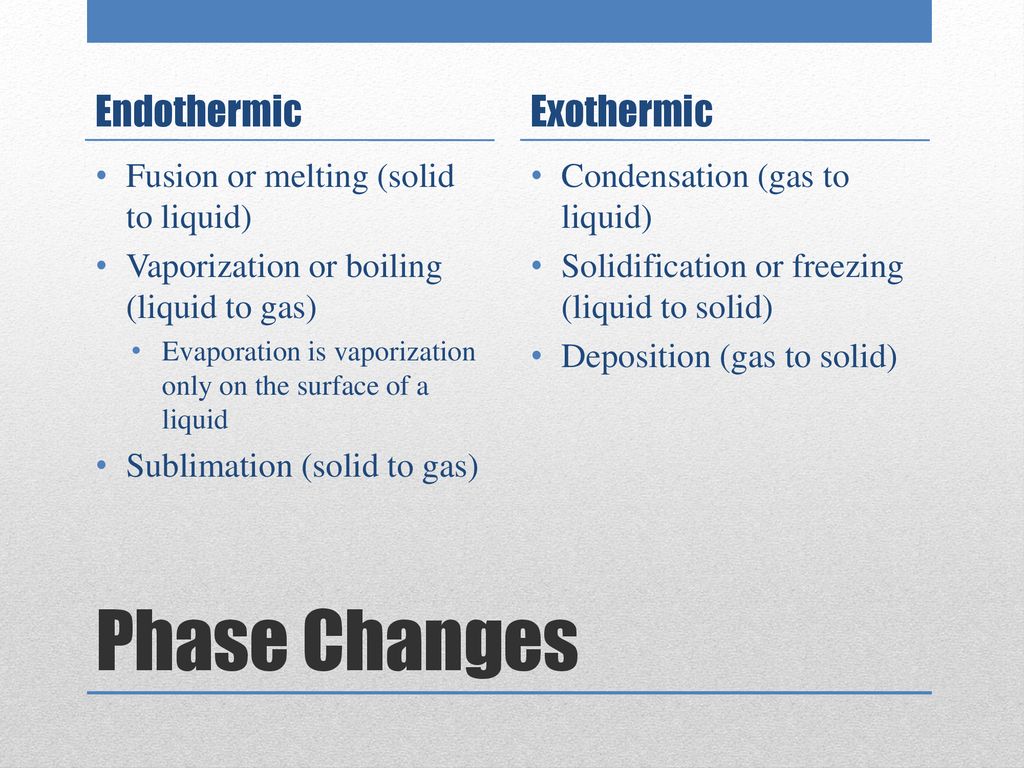
Endothermic processes are like: * Melting (solid to liquid): Ice cream cone melting on a hot day. Needs energy to become goopy! * Vaporization (liquid to gas): Boiling water. Needs energy to become steam. * Sublimation (solid to gas): Dry ice disappearing. Needs a LOT of energy to go straight to gas.
Exothermic processes are like: * Freezing (liquid to solid): Water turning into ice. Releases energy as it gets rigid. * Condensation (gas to liquid): Fog forming. Releases energy as gas molecules huddle up. * Deposition (gas to solid): Frost forming. Releases energy as gas molecules become solid.
It’s all about the energy exchange. Endothermic needs energy in, exothermic gives energy out. Think of it as a cosmic energy loan program. Some phase changes are borrowing (endothermic), and some are paying back with interest (exothermic).
Next time you see steam rising, ice melting, or dew forming, you can impress your friends (or just yourself!) by knowing exactly what kind of energy party those molecules are having. It’s a little peek into the energetic hustle and bustle of the universe, happening all around us, all the time. Pretty neat, right?
