Classify The Following Line-bond Formulas As Ketones Or Aldehydes
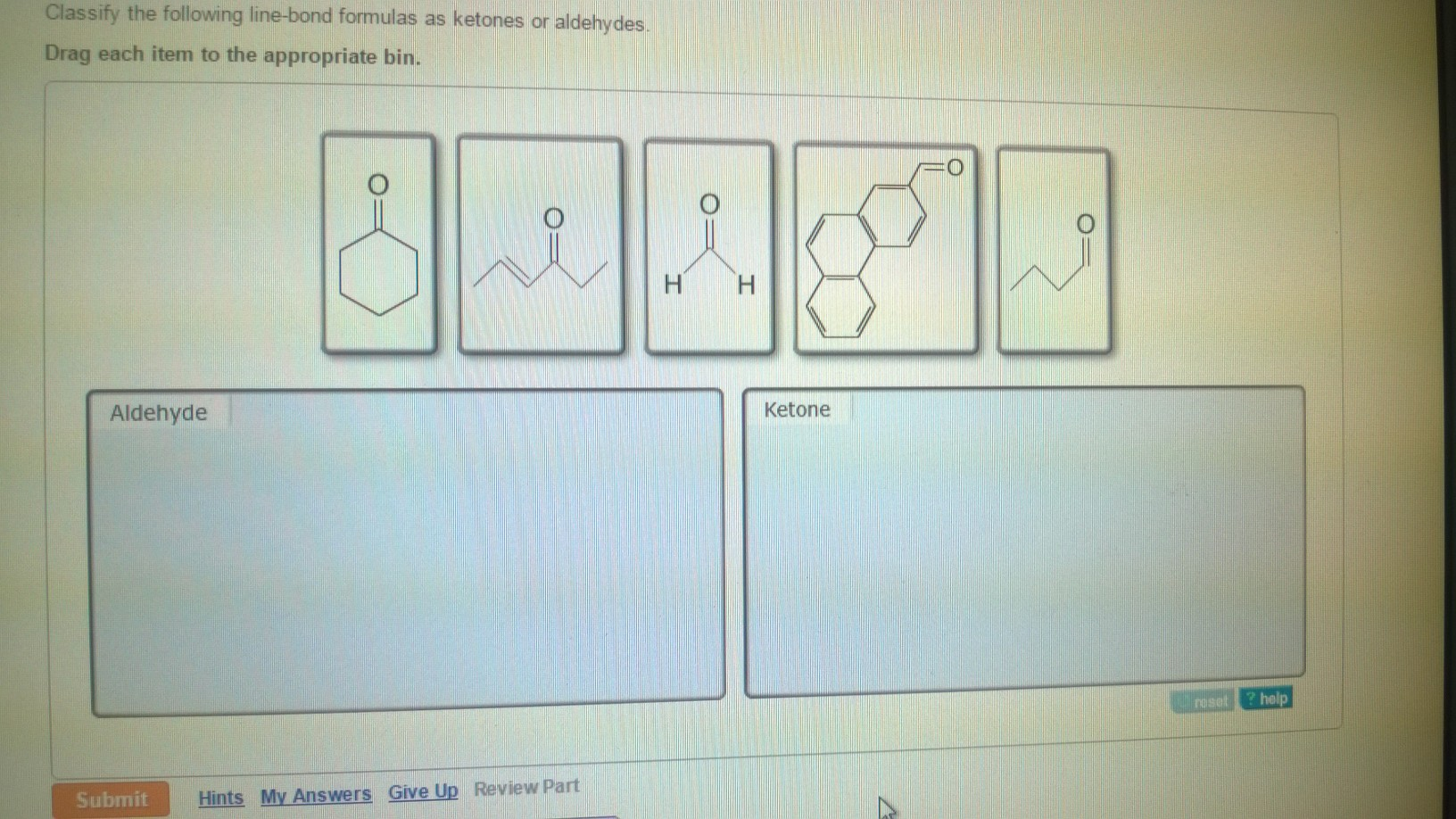
Hey there, my fellow chemistry adventurers! So, you've been staring at these funky line-bond formulas, scratching your head, and wondering, "Are these guys ketones or aldehydes?" Don't sweat it! We've all been there. It's like trying to figure out if your friend is a cat person or a dog person just by looking at their drawing of a fuzzy creature. But fear not, because today we're going to crack this code together, in a way that's so easy, you'll feel like you've unlocked a secret level in a video game. And let's be honest, who doesn't love a good secret level?
Think of organic chemistry like a big, exciting puzzle. We’ve got these building blocks, these molecules, and each one has its own personality, its own special feature. Today, our spotlight is on a very specific feature: the carbonyl group. This is basically a carbon atom with a double bond to an oxygen atom. Think of it as the molecule's "face" – it's what gives it a lot of its character and reactivity. It looks like this: C=O. Simple, right? It's the superhero cape of our molecules!
Now, the magic, or should I say, the chemistry, happens when this carbonyl group decides to hang out with its buddies, the carbon and hydrogen atoms. Depending on who the carbonyl group is hanging out with, it can be either an aldehyde or a ketone. It’s like deciding whether to have pizza or tacos for dinner – both are delicious, but they’re definitely different!
So, how do we tell them apart? It all comes down to the neighbors of our carbonyl carbon. Imagine the carbonyl carbon is the star of a party. The aldehydes and ketones are distinguished by the guests sitting right next to the star.
Aldehydes: The "Always on the Edge" Crew
Let’s talk about our aldehydes first. These guys are the life of the party, but they always like to be on the edge. Literally. The carbonyl carbon in an aldehyde is always attached to at least one hydrogen atom. Picture this: the carbonyl carbon (our C=O) is holding hands with a hydrogen atom on one side, and on the other side, it's either holding hands with another hydrogen atom or with a carbon chain (which, if you remember, is a bunch of carbons and hydrogens all linked up). So, the general formula looks something like R-CHO, where 'R' can be a hydrogen atom (H) or a carbon chain.
Think of it like this: the aldehyde is the friendly neighbor who always has their porch light on. The carbonyl group is right there, at the end of the line, easily accessible. It's not hiding in the middle of the street, is it? Nope, it's right there, at the front door.
Why is this "on the edge" thing so important? Well, those hydrogen atoms attached to the carbonyl carbon are quite special. They make aldehydes super reactive. They're like little buttons that you can press to make exciting chemical reactions happen. They’re eager to get involved, ready to share their hydrogen atoms in a chemical handshake.
Let’s look at an example. If you see a line-bond formula where the carbonyl group (C=O) is at the very end of a chain, and there’s a little "zig" or "zag" pointing away from it (which represents a carbon chain) or even just another line that clearly ends there, and that carbonyl carbon also has a hydrogen attached (sometimes explicitly shown, sometimes implied), you’re looking at an aldehyde. The key is that carbonyl carbon is only bonded to one other carbon atom (or zero if it's formaldehyde, which is just H-CHO, the simplest aldehyde of all – the ultimate minimalist!).

It’s like a… a pointing finger! The carbonyl group is pointing outwards, saying, "Hey! Look at me! And see this hydrogen? It’s ready for action!"
Let's try to visualize it. Imagine a skeletal structure. You'll see a line representing a carbon chain. At the very tip of one of these lines, you'll spot a C double-bonded to an O. If that carbon at the tip also has a hydrogen attached (or if it's at the end of the line and you know it needs four bonds, and only has one other bond to a carbon, the other two must be to hydrogens), bingo! Aldehyde!
Sometimes, they draw it out a bit more explicitly, showing the 'H' attached to the carbonyl carbon. That’s like a giant neon sign saying, "ALDEHYDE HERE!" But even if the 'H' isn't explicitly drawn, the position at the end of the chain is a dead giveaway, especially if the carbon has only one other bond to a carbon atom.
Ketones: The "In the Middle" Masterminds
Now, let's switch gears to our ketones. These guys are a bit more… reserved. They like to be in the middle of things, not on the edge. In a ketone, the carbonyl carbon is bonded to two other carbon atoms. No hydrogens are directly attached to the carbonyl carbon in a ketone. It’s like the carbonyl group is having a cozy chat with two carbon friends, completely surrounded.
The general formula for a ketone is R-CO-R', where 'R' and 'R'' are both carbon chains (or rings, but let's keep it simple for now!). The 'CO' part is our carbonyl group, sitting pretty in the middle.
Think of the ketone as the popular kid who's always the center of attention in the group photo. The carbonyl carbon is the one in the middle, with a carbon friend on its left and a carbon friend on its right. It's not reaching out to the edge; it's firmly established in the heart of the molecule.
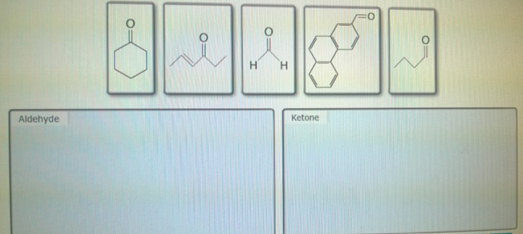
Because ketones don't have those reactive hydrogen atoms directly attached to the carbonyl carbon, they are generally a bit less reactive than aldehydes. They’re more stable, less eager to jump into every single chemical reaction. They’re the ones who listen more than they talk at the party, but they still have their own cool vibe.
When you're looking at a line-bond formula, you're looking for that C=O group. If that carbonyl carbon is bonded to two other carbon atoms (represented by lines branching off, or by being part of a carbon chain that continues on both sides), then you've got yourself a ketone. The key here is that the carbonyl carbon is not at the end of a chain, and it's definitely not bonded to a hydrogen.
It’s like a delicious sandwich! The carbonyl group is the filling, and the bread slices on either side are the carbon chains. Yum! And definitely not on the edge.
So, let’s recap the super-secret handshake for identifying them. Look at the carbon atom that’s double-bonded to the oxygen (the carbonyl carbon).
The Grand Unveiling: How to Classify!
Okay, time for the moment of truth! Grab your imaginary magnifying glass and let’s examine these line-bond formulas. It’s like being a detective, and the carbonyl carbon is your prime suspect!
Step 1: Find the Carbonyl Group (C=O). This is your beacon of hope, your guiding star. It's that carbon atom with the double bond to oxygen. If you don't see a C=O, you're looking at a different functional group altogether. Maybe a super-exciting alcohol or a mischievous alkane, but definitely not an aldehyde or ketone. So, first things first, find that double bond!
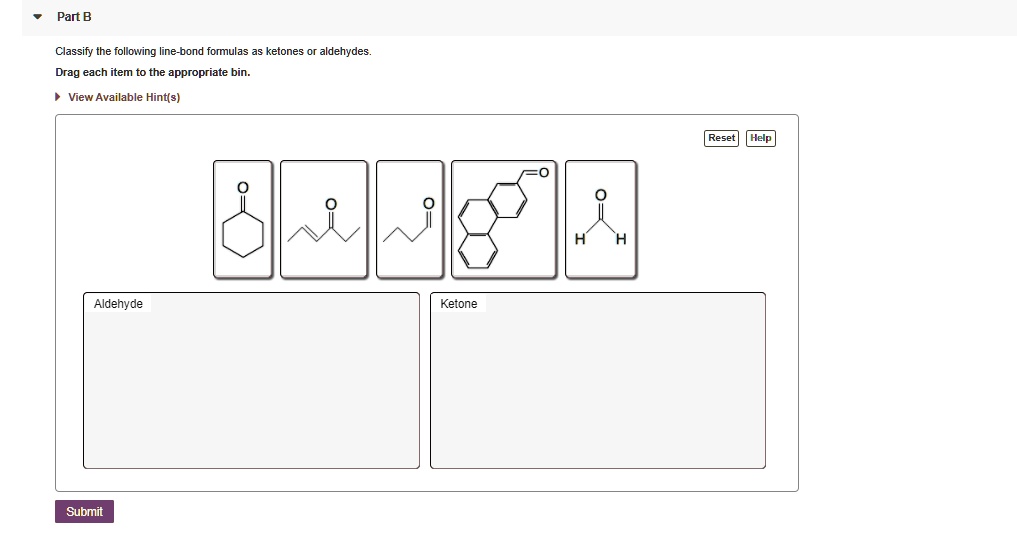
Step 2: Check the Neighbors of the Carbonyl Carbon. This is where the magic happens! Look at the atoms directly attached to the carbonyl carbon.
- If the carbonyl carbon is attached to at least one hydrogen atom (H), AND the other attachment is either another hydrogen or a carbon chain: You've got an ALDEHYDE! Remember, aldehydes like to be on the edge. The presence of that hydrogen makes them extra special and reactive. Sometimes the hydrogen isn't drawn, but if the carbonyl carbon is at the end of a chain and only bonded to one other carbon, it's still an aldehyde. The universe insists it has those two hydrogens to satisfy its bonding needs!
- If the carbonyl carbon is attached to TWO carbon atoms (and NO hydrogen atoms): You've got a KETONE! Ketones are the chill ones, happy to be in the middle, surrounded by their carbon buddies.
It's really that simple! Think of it as a simple rule: Is there a hydrogen directly attached to the carbonyl carbon? Yes? Aldehyde. No? Ketone (as long as it's bonded to two carbons, of course!).
Let's try a little practice, shall we? Imagine you see this:
A line ending in a C=O, with a line branching off from the C in C=O. That C in C=O is at the end of a chain. It must have a hydrogen attached to make four bonds. So, that's an aldehyde!
Now imagine this:
A line, then a C=O, then another line. The C in C=O is clearly connected to a carbon on its left and a carbon on its right. No hydrogens are attached to it. That's a ketone!
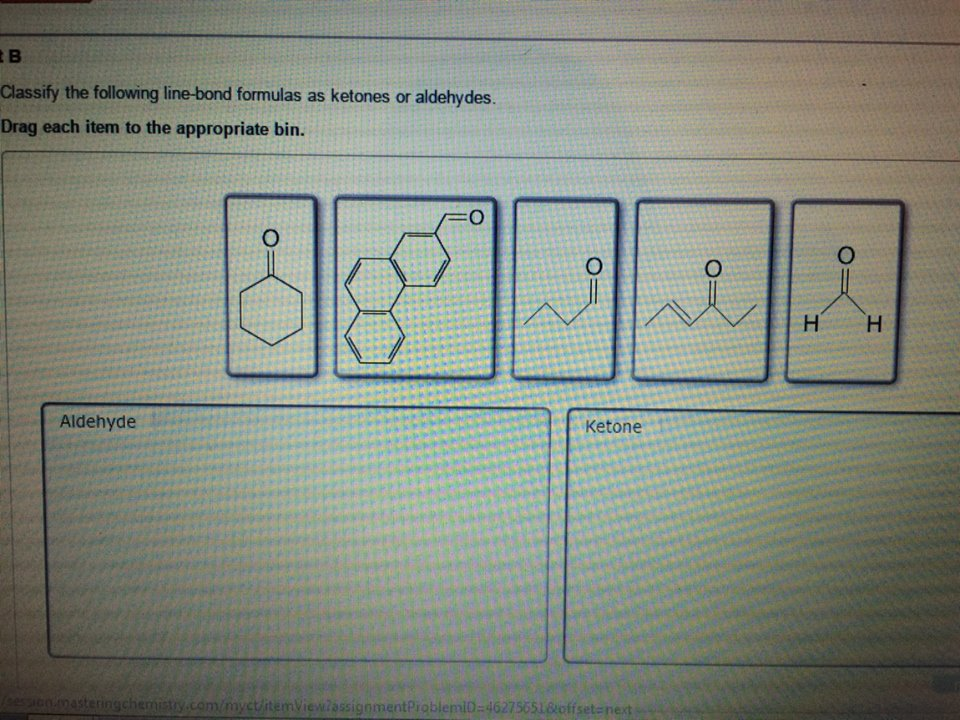
What if you see a hexagon shape, and one of the corners has a C=O attached to it? That hexagon corner is a carbon atom. It's bonded to two other carbon atoms within the hexagon. So, that C=O is bonded to two carbons. Ketone!
And if you see a zig-zag chain, and right in the middle of the zig-zag, there’s a C=O. That carbonyl carbon is connected to a carbon before it and a carbon after it. Again, no hydrogens. Ketone!
It’s like playing “I Spy” with molecules! You spy the C=O, then you spy its neighbors. And then, poof, you've got your answer!
Don't be fooled by the fancy zig-zags and triangles. The principle remains the same. It's all about what's directly connected to that carbonyl carbon. It’s the molecule’s way of saying, "Here I am, this is what I do!"
And hey, if you ever get mixed up, just remember the simple rule: Aldehydes are on the edge with a hydrogen friend, ketones are in the middle with carbon friends. Easy peasy lemon squeezy, right?
So, the next time you're faced with a line-bond formula and a question mark about its identity, take a deep breath, channel your inner detective, and remember our little chat. You've got this! You're not just looking at lines and dots anymore; you're seeing the personalities, the unique features, the very essence of these molecules. Each one is a tiny, fascinating world, and you're learning to understand their language. Keep exploring, keep questioning, and keep that curiosity alive. You're on an amazing journey of discovery, and every molecule you decipher is a step further into a wonderful, complex, and ultimately beautiful universe. So go forth, my friends, and classify with confidence and a smile! The world of chemistry is waiting for your keen eye and brilliant mind!
