Classify The Bonds As Ionic Polar Covalent Or Nonpolar Covalent

Imagine all the things you love in life – your comfy couch, that perfectly brewed cup of coffee, your best friend’s contagious laugh. What do they all have in common? They’re all built from tiny, invisible building blocks that love to stick together! These little guys are called atoms, and when they decide to team up and share or swap their tiny bits of electricity (called electrons), they create something called a chemical bond. It’s like a super-secret handshake that holds the universe together, from the sparkle in your diamond ring to the fizzy bubbles in your soda.
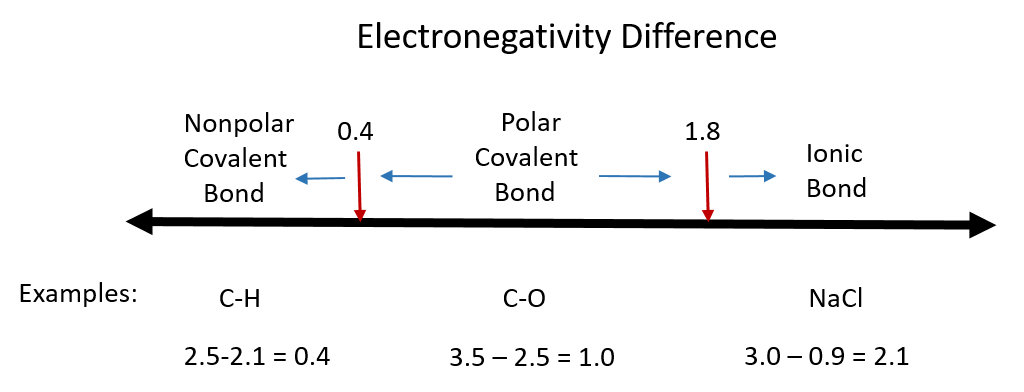
Now, these atom partnerships aren’t all the same. Some are like a really strong hug, where one atom basically gives all its electrons away to another. This is like when your younger sibling is begging you for your favorite toy, and you finally just give it to them so they’ll stop whining. This kind of super-clingy relationship is called an ionic bond. It’s all about a complete transfer, a bold declaration of “You’ve got it now!”
Think about salt, that everyday miracle that makes popcorn sing. When sodium (a grumpy atom that really wants to get rid of an electron) meets chlorine (a greedy atom that desperately wants an electron), they form a perfect ionic bond. Sodium is like the generous friend who always offers to pay for the first round, and chlorine is the one who happily accepts. This creates the crunchy, delicious crystals of table salt we know and love. It’s a beautiful, if slightly unequal, partnership.
But not all atom relationships are so one-sided. Sometimes, atoms are more like roommates who decide to share their stuff. They’re not giving it all away, nor are they holding on super tight; they’re just saying, “Let’s work this out together.” This is where we get into the world of covalent bonds. It’s a more equitable arrangement, a mutual understanding of sharing.
Now, even in this sharing economy of electrons, there can be a little bit of drama. Some atoms are just naturally a bit more persuasive, a bit more… well, electronegative. They have a stronger pull on those shared electrons, like a magnet that’s just a tiny bit stronger than the other. When this happens, the sharing isn’t perfectly equal. It’s like when you’re sharing a pizza, but one person always manages to snag the slice with the most pepperoni. This kind of slightly lopsided sharing creates a polar covalent bond.

Water, the most essential molecule on Earth, is a fantastic example of polar covalent bonds. An oxygen atom is like the parent who wants things just so, and the two hydrogen atoms are like the kids who are happy to go along with it. The oxygen pulls the shared electrons a little closer, giving it a slightly negative charge, while the hydrogens get a slightly positive charge. This gentle tug-of-war is what makes water molecules stick to each other, creating the surface tension that lets water striders skate on ponds and allows us to have those refreshing showers.
It’s this slight unevenness that makes water so amazing. It’s like a group of friends who have slightly different personalities, but when they’re together, they create a vibrant, dynamic group. This polarity in water is the reason it can dissolve so many things, acting like a universal solvent for life. Without these slightly unequal friendships between atoms, our world would be a very different, and much less interesting, place.

On the other hand, some atom pairs are the epitome of fairness. They’re like best friends who split everything exactly down the middle. When two atoms of the same kind decide to bond, or when two atoms with very similar electron-pulling power get together, they share their electrons so equally that you can’t tell who has the slight edge. This is a nonpolar covalent bond. It’s pure, unadulterated equality.
Think about the air we breathe. Oxygen gas, the stuff that keeps us alive, is made of two oxygen atoms bonded together. Since they are identical, they have absolutely no reason to favor one over the other when it comes to those precious electrons. The sharing is perfect, the bond is balanced. It’s a harmonious partnership, just two identical twins deciding to share their toys.

Another great example is the oil that makes your salad dressing so delicious. Fats and oils are made up of long chains of carbon and hydrogen atoms. Carbon and hydrogen are pretty similar in how they attract electrons, so the bonds between them are very nonpolar. This is why oil and water don’t mix – they’re like two different social circles that just don’t have much in common, lacking the slight electrical attraction needed to bridge the gap.
So, next time you’re enjoying a meal, taking a sip of water, or just breathing in the fresh air, remember the incredible world of atomic partnerships happening all around you. You’ve got the generous givers and takers in ionic bonds, forming the solid structures of things like salt. You have the slightly pushy sharers in polar covalent bonds, creating the magic of water. And you have the perfectly balanced buddies in nonpolar covalent bonds, making up essential gases and oils.
It’s a fascinating dance of electrons, a constant give-and-take that builds everything we see and experience. These bonds aren’t just abstract scientific concepts; they are the fundamental reason why your favorite sweater is soft, why the sun shines, and why life itself is possible. They are the unsung heroes of our everyday existence, and understanding their subtle differences can give you a whole new appreciation for the world around you.
Isn’t it amazing that something so small and invisible can have such a profound impact on our lives? From the salty sprinkle on your fries to the water that quenches your thirst, each bond tells a unique story of connection and interaction. It's a reminder that even in the smallest particles, there's a universe of fascinating relationships waiting to be discovered.
