Classify Each Reaction According To Whether A Precipitate Forms.
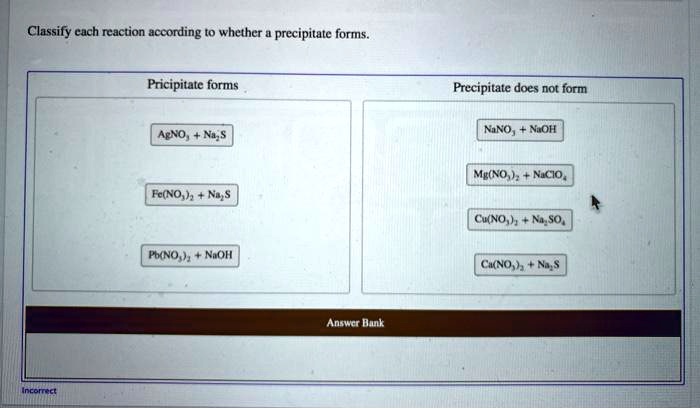
Alright, buckle up, science enthusiasts (and even you curious folks who just stumbled in)! We're about to dive into the sparkly, sometimes murky, world of chemical reactions. And guess what? We're not just looking at any old mix-and-match. Oh no, we're playing a thrilling game of "Did it Make a Mess?" or, in fancier terms, classifying reactions based on whether a precipitate decides to show up. Think of it like hosting a party for tiny molecules – sometimes, things get a little too exciting, and you end up with something solid forming at the bottom of the punch bowl. That, my friends, is our star player today: the magnificent, the sometimes-annoying, the utterly fascinating precipitate!
The Party's Started! What's Happening Here?
Imagine you're a master chef, whipping up some culinary magic in your lab-kitchen. You're mixing liquids, you're dissolving things, you're feeling the vibe. Suddenly, POW! Something solid appears out of nowhere, floating around in your perfectly clear liquid. It's like a surprise guest at your molecular get-together, and this guest has decided to crash the party by becoming a solid. We call this the formation of a precipitate. It’s basically a chemical reaction saying, "Hey, these two liquids just didn't want to play nice, so they decided to get jiggy and make something new and solid!"
Now, not all reactions are this dramatic. Some are like a gentle whisper, where everything stays perfectly blended, a harmonious symphony of dissolved molecules. Others are like a loud bang, where a visible, solid chunk forms. Our mission, should we choose to accept it (and we totally do!), is to figure out which is which. It's like being a detective, looking for clues in our chemical concoctions. Is there a solid hiding where there wasn't one before? Eureka! We've got ourselves a precipitation reaction!
Let's think about it this way: you're making lemonade. You mix lemon juice and water. Perfect! Everything stays dissolved. That's a reaction where no precipitate forms. Now, imagine you accidentally grabbed the salt shaker instead of the sugar. You dump a bunch of salt into your water. What happens? You'll see those little salt crystals sinking to the bottom, not completely dissolving anymore. That, my friends, is a classic example of a precipitate forming. The salt molecules decided they'd rather hang out with each other in solid form than stay mixed with the water. Talk about a social butterfly that prefers its own kind!
The "No Solids Allowed!" Club
On the flip side, we have reactions where the molecules are the ultimate chillers. They mingle, they mix, they become one happy, dissolved family. Think about dissolving sugar in your tea. The sugar crystals disappear, leaving your tea sweet and clear. That's a reaction where the sugar molecules are perfectly happy staying in their dissolved state. No solid leftovers, no chunky surprises. This is the "no solids allowed" club, and frankly, sometimes it's the most satisfying outcome, like a perfectly smooth smoothie. We call these reactions non-precipitation reactions. They're the unsung heroes of the chemical world, keeping things tidy and transparent.
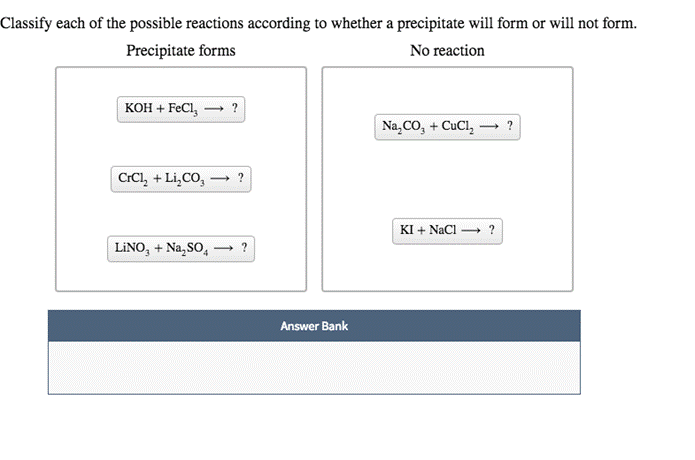
Another example? When you mix vinegar and baking soda, you get fizzing! That fizzing is actually a gas escaping, not a solid forming. So, even though there's a lot of action, it's not a precipitate. The gas bubbles are like little party poppers, celebrating the new substances formed, but they eventually dissipate. We're looking for something that settles down, something that sticks around as a solid, like a particularly stubborn stain on your favorite shirt.
Spotting the Solid: Your Secret Weapon
So, how do we become precipitate-spotting pros? It's all about observation, my dear Watson! When you're mixing two clear liquids and suddenly you see tiny specks, a cloudiness, or a chunky deposit at the bottom, that's your cue. You've witnessed the birth of a precipitate. It's like seeing a tiny, solid snowflake form in your drink, except it's made of entirely new chemical compounds. It's a visual confirmation that a precipitation reaction has occurred.
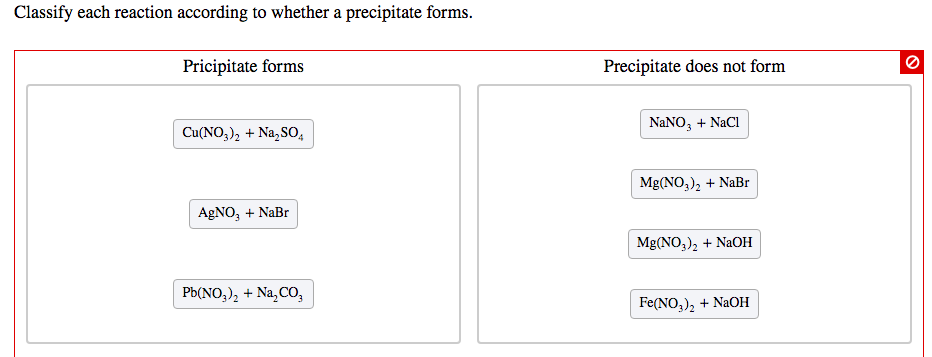
Think of it like this:
- Clear liquid + Clear liquid -> Cloudy mess with settled bits? BIG YES! That's a precipitation reaction!
- Clear liquid + Clear liquid -> Still perfectly clear? ALL GOOD! That's a non-precipitation reaction!
It's genuinely that simple! We're not asking you to memorize complex chemical formulas (unless you're secretly a chemist-in-training, in which case, go you!). We just want you to be aware of the visual evidence. If you see a solid forming where there wasn't one before, you've nailed it. You've classified it! You're basically a chemical fortune teller, predicting the solid future of your mixtures.
So, next time you're playing around with liquids (safely, of course!), or even just watching a science show, keep your eyes peeled. Are those molecules getting a bit too cozy and forming something solid? Or are they keeping it cool and staying dissolved? The answer will tell you everything you need to know. It’s a simple classification, but it unlocks a whole world of understanding about how these tiny building blocks of our universe interact. Embrace the solid, celebrate the clear, and have fun with your chemical sleuthing! You're doing great!
