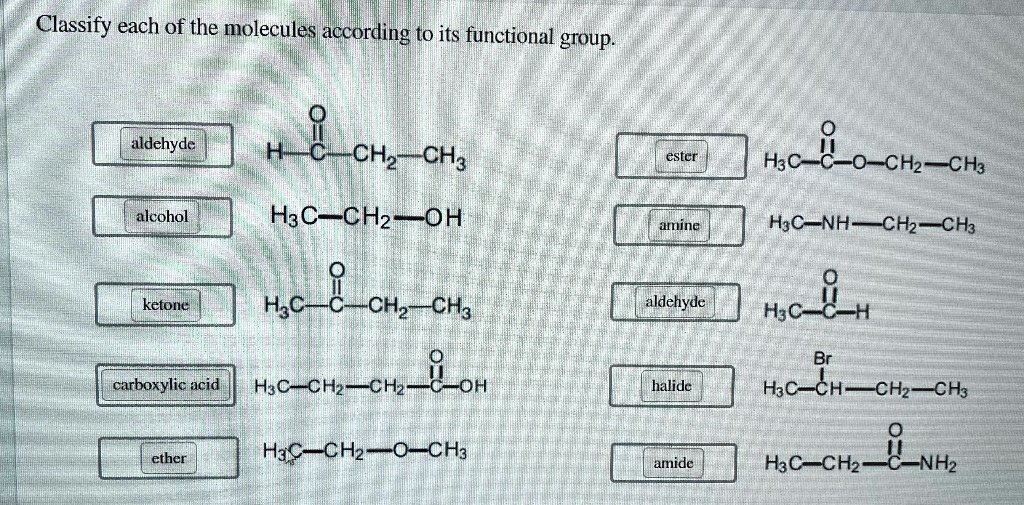
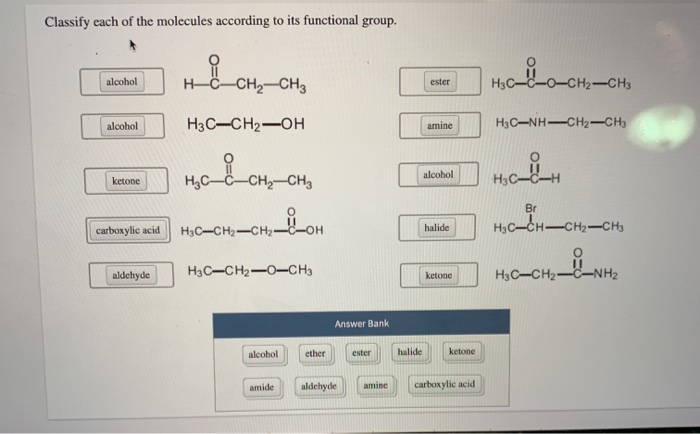
Classify Each Of The Molecules According To Its Functional Group
Hey there, coffee buddy! Grab your mug, settle in. Today, we're diving into something that might sound a tad intimidating at first – functional groups. But honestly, it's more like learning to recognize different "personalities" of molecules. Think of it like this: you've got your bestie who's always cracking jokes, your chill friend who just goes with the flow, and maybe that one person who’s super organized. Molecules are kinda like that, but with, you know, atoms and bonds. And once you get the hang of it, classifying them is a piece of cake. Seriously!
So, what exactly are these "functional groups," anyway? Imagine a molecule is like a LEGO castle. You've got the basic bricks, the carbon and hydrogen skeleton, right? That's the backbone. But then, you add these special, often more reactive, bits. These bits are the functional groups! They're the cool turrets, the fancy flags, the little drawbridges. They're what give the molecule its oomph, its characteristic behavior, and its name, often. Without them, most organic molecules would be pretty boring, like a plain beige wall. Yawn.
It’s all about recognizing these specific arrangements of atoms. It's like spotting your favorite brand of coffee from across the room. You just know it. And the cool thing is, once you learn a few common ones, you can start to predict how a molecule will behave. Like, "Oh, that guy has an –OH group? Probably going to be a bit polar, maybe soluble in water, and might do some interesting reactions." It's like a superpower, almost!
Let’s start with the absolute OG of functional groups, the ones you'll see everywhere. First up, we’ve got the alcohols. What’s their signature move? They have a special little attachment: an –OH group. Yep, that’s a hydrogen atom hanging out with an oxygen atom, and that whole unit is stuck to the carbon chain. Think of ethanol, the stuff in, well, that drink you might enjoy sometimes (responsibly, of course!). Or even water, H2O, technically has an –OH, though it’s a bit of a special case. Alcohols are often polar, meaning they have a slight positive and negative end, which makes them good at dissolving other polar things, like sugar. So, your sugar dissolves in your coffee because of those polar –OH groups!
Now, what happens if you take that –OH group and slap it onto a carbon that’s also got a double bond to an oxygen? BOOM! You get a whole new family: aldehydes and ketones. These guys are related, but they have a little difference in where that double-bonded oxygen is hanging out. In aldehydes, the C=O group is at the end of a carbon chain. Think of formaldehyde, which is, uh, used for preserving things. Not exactly for drinking! Ketones, on the other hand, have the C=O group in the middle of the carbon chain. Acetone, that stuff nail polish remover is made of? That’s a classic ketone. They're both pretty reactive and can be used to make all sorts of cool stuff.

Speaking of the C=O group, it’s like the VIP of functional groups. It can be part of many different families. Let’s keep rolling with it. What if that C=O group decides to hang out with an –OH group, but this time, the –OH is attached to the same carbon as the C=O? This is where we get carboxylic acids. These guys are acidic, hence the name! They can donate a hydrogen ion (H+) pretty easily. Think of vinegar, also known as acetic acid. That tangy taste? That’s your carboxylic acid at work. They’re super important in biology, too, like in amino acids, the building blocks of proteins. Mind. Blown.
Okay, let’s twist it again. What if, instead of an –OH, that C=O group is attached to a nitrogen atom? Now we’re talking about amides. Amides are like the super-stable cousins of carboxylic acids. They’re found all over the place in nature, most famously in proteins. Those peptide bonds that link amino acids together? They’re amide bonds! So, the very structure of your muscles and enzymes relies on these little amide groups. Pretty wild, right? They’re not as acidic as carboxylic acids, which makes them a bit more mellow. They’re like the dependable middle child of the carbonyl family.
Let’s switch gears a bit. We've been playing with oxygen a lot. What about nitrogen? We’ve seen it in amides, but it can also be on its own, just chillin’ with some carbons. These are amines. Amines are basically ammonia (NH3) where one or more of the hydrogens has been replaced by a carbon group. They're often basic, meaning they can accept a proton. Think of neurotransmitters like dopamine and serotonin – those are amines! They play a huge role in your brain and nervous system. So, the next time you’re feeling happy, you can thank an amine (or a bunch of them!). They can also smell…interesting. Some small amines have a distinctly fishy odor. Charming!
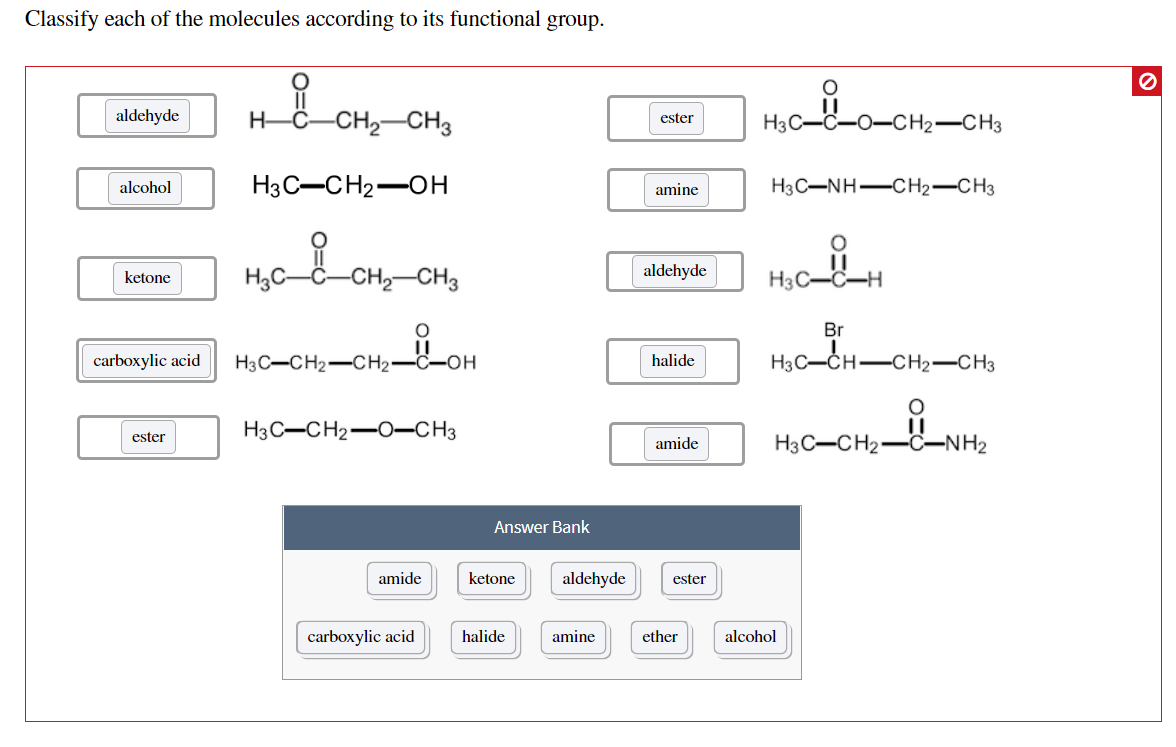
Now, let’s talk about a group that’s all about sharing electrons: ethers. Ethers are like having two carbon chains connected by an oxygen atom. So, it’s R-O-R, where R is a carbon chain. Think of diethyl ether, which used to be used as an anesthetic. It’s pretty volatile, meaning it evaporates easily. They’re less polar than alcohols, so they’re not as good at dissolving in water. They're more like the aloof relatives who just observe. They’re generally pretty unreactive, which can be a good thing sometimes. Like a really chill roommate who never borrows your stuff.
What about those guys that look like they have a little ring of double bonds? Those are aromatic compounds, and benzene is the poster child. Benzene is a six-carbon ring with alternating double and single bonds. It’s super stable because those electrons are delocalized, meaning they’re not stuck in one place. It's like a free-spirited electron commune. Aromatic compounds are everywhere, from gasoline to dyes to pharmaceuticals. They often have a distinct smell, hence the "aromatic" part. Just be careful, some aromatic compounds can be pretty toxic, so it’s not all roses and pleasant perfumes.
Let’s get back to the C=O party, but this time, let’s invite halogens. When you have a carbon atom bonded to a halogen (like fluorine, chlorine, bromine, or iodine), you get a halide, or more specifically, an alkyl halide if it's attached to a carbon chain. Think of chloromethane, CH3Cl. These guys are super useful as intermediates in chemical reactions. They’re like the little worker bees of organic chemistry, ready to be transformed into something else. They can be pretty reactive, and some are used as solvents or refrigerants. Just be aware that some can be pretty nasty stuff, so handle with care!

Now, let’s talk about something that’s become really important in recent years: esters. Esters are formed when a carboxylic acid reacts with an alcohol. They have that C=O group, and then an –O–R group attached to the same carbon. Lots of fruits get their yummy smells from esters! So, if something smells like bananas or strawberries, chances are it’s an ester at play. They’re also used in plastics and as solvents. They’re the sweet-smelling, versatile members of the carbonyl family. They’re like the perfumers of the molecular world.
We’ve covered a lot of ground, right? But there are even more families out there. Think about molecules that have a triple bond between two carbon atoms – those are alkynes. They’re pretty reactive, those triple bonds are itching to be broken and rearranged. And then there are alkenes, which have a double bond between two carbon atoms. They're also reactive, but a bit less so than alkynes. These are the simple hydrocarbons, the foundation for a lot of organic chemistry. They’re like the sturdy building blocks of the molecular universe, just waiting for their functional group decorations.
So, how do you actually classify these things? It’s like being a detective. You look at the molecule, and you scan it for those characteristic bits. Does it have an –OH? Bingo, alcohol. Is there a C=O at the end of a chain? Hello, aldehyde! See that C=O in the middle? That’s a ketone. Is there a C=O next to an –NH2? Amide, for sure. You’re looking for patterns, for those familiar arrangements of atoms that scream out their identity. It’s like recognizing faces in a crowd, but with molecules.
Sometimes, a molecule can have more than one functional group. That’s when things get really interesting! Think of amino acids, which have both an amine group and a carboxylic acid group. They’re like molecules with dual personalities, capable of doing all sorts of cool chemistry. It’s like a molecule that can both tell jokes and organize your sock drawer. Multitalented!
The key is to practice. The more molecules you look at, the more familiar these functional groups will become. You'll start to spot them instantly. It’s like learning a new language. At first, it’s all about memorizing vocabulary, but then, you start to understand the grammar, the sentence structure, and suddenly, you can have a whole conversation. And with molecules, that conversation is about how they react, how they interact, and what amazing things they can do.
Don't get discouraged if it feels a bit overwhelming at first. Even the most seasoned chemists started at square one. Just take it one functional group at a time. Master the alcohols, then move on to the aldehydes and ketones. Each one is a little puzzle piece, and eventually, you’ll see the whole picture. And when you do, you’ll realize that the world of organic chemistry isn't some scary, impenetrable fortress. It’s just a bunch of molecules with different, fascinating personalities, all hanging out and doing their thing. Pretty neat, huh? Now, about that second cup of coffee…
