Choose Reagents To Convert 2-cyclohexenone To The Following Compounds.
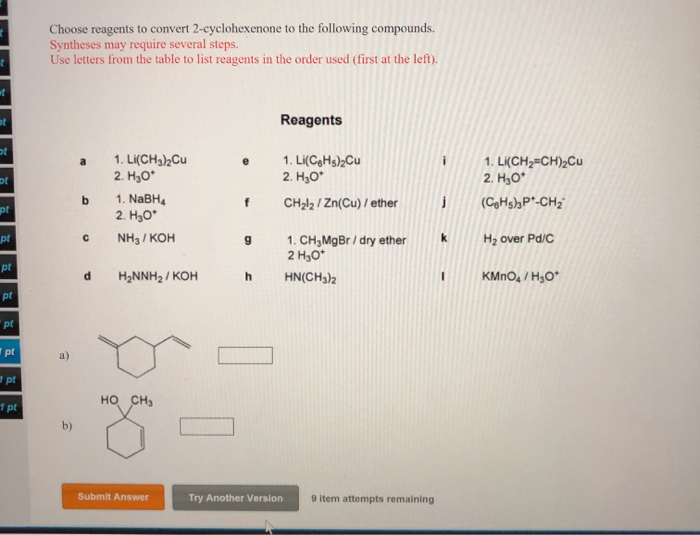
Welcome to the exciting world of molecular transformations! Have you ever wondered how scientists can take one molecule and skillfully turn it into another, often creating entirely new and useful substances? It’s like a culinary adventure, but with tiny building blocks instead of ingredients. Today, we're diving into a specific, yet wonderfully illustrative, example: converting 2-cyclohexenone into a couple of fascinating new compounds. Don't worry if chemistry sounds intimidating; we're going to keep it light, fun, and focus on the "what" and "why" rather than getting bogged down in the nitty-gritty details. Think of this as a peek behind the curtain of how chemists design reactions to achieve specific goals.
The Magic of Chemical Change
Why is this kind of transformation so popular and useful? Well, imagine you're a chemist with a specific need. Perhaps you're trying to synthesize a new drug, develop a more effective plastic, or even create a special scent for a perfume. Often, the perfect molecule isn't readily available. That's where reactions like the ones we'll explore come in. They are the bridge between what we have and what we want. 2-cyclohexenone is a fantastic starting point because it has a bit of everything – a ring structure (the 'cyclohex' part), a double bond (the 'en' part), and a ketone group (the 'one' part). This versatility makes it a prime candidate for a variety of chemical adventures. By carefully choosing the right tools – the reagents – we can selectively change specific parts of the molecule, leading to entirely new structures with different properties.
Unlocking New Possibilities with 2-Cyclohexenone
Our starting molecule, 2-cyclohexenone, is a six-membered ring with a double bond and a carbonyl group (C=O) situated next to that double bond. This arrangement is special and allows for several types of reactions. We’re going to explore how to transform it into two distinct compounds. Each transformation requires a unique set of reagents, which are the chemical substances that drive the reaction. Think of reagents as the specialized knives and whisks a chef uses to prepare ingredients.
Scenario 1: Creating a Saturated Ring System
Our first goal is to transform 2-cyclohexenone into cyclohexanone. This might seem like a small change, but it’s significant! We’re essentially getting rid of the double bond. How do we do that? We need to "saturate" the ring. This is like adding extra atoms to fill in the gaps created by the double bond. The most common and effective way to achieve this is through a process called hydrogenation.

To convert 2-cyclohexenone to cyclohexanone, we would typically use hydrogen gas (H₂) in the presence of a metal catalyst. Common catalysts include palladium (Pd), platinum (Pt), or nickel (Ni). The catalyst helps to break the H-H bond in hydrogen gas, allowing the hydrogen atoms to add across the double bond of 2-cyclohexenone. This process effectively removes the double bond and replaces it with single bonds, resulting in the saturated ring of cyclohexanone. It's a straightforward and very useful reaction in organic chemistry.
Why is this useful? Cyclohexanone itself is a major industrial chemical, used extensively as a precursor in the production of nylon. So, this simple transformation is a stepping stone to creating essential materials we use every day. It demonstrates how we can selectively target and modify specific functional groups within a molecule.
Scenario 2: Adding Functionality to the Double Bond
Now, let's move on to our second exciting transformation. This time, we want to keep the double bond, but we want to add something to it. Specifically, we're aiming to create 2-cyclohexen-1-ol. Notice the "-ol" ending? That signifies an alcohol group (-OH). We’re taking our 2-cyclohexenone and changing the carbonyl group (C=O) into a hydroxyl group (C-OH), while keeping the double bond intact. This is a classic example of a reduction reaction, where we’re essentially adding hydrogen and breaking the carbon-oxygen double bond of the ketone.
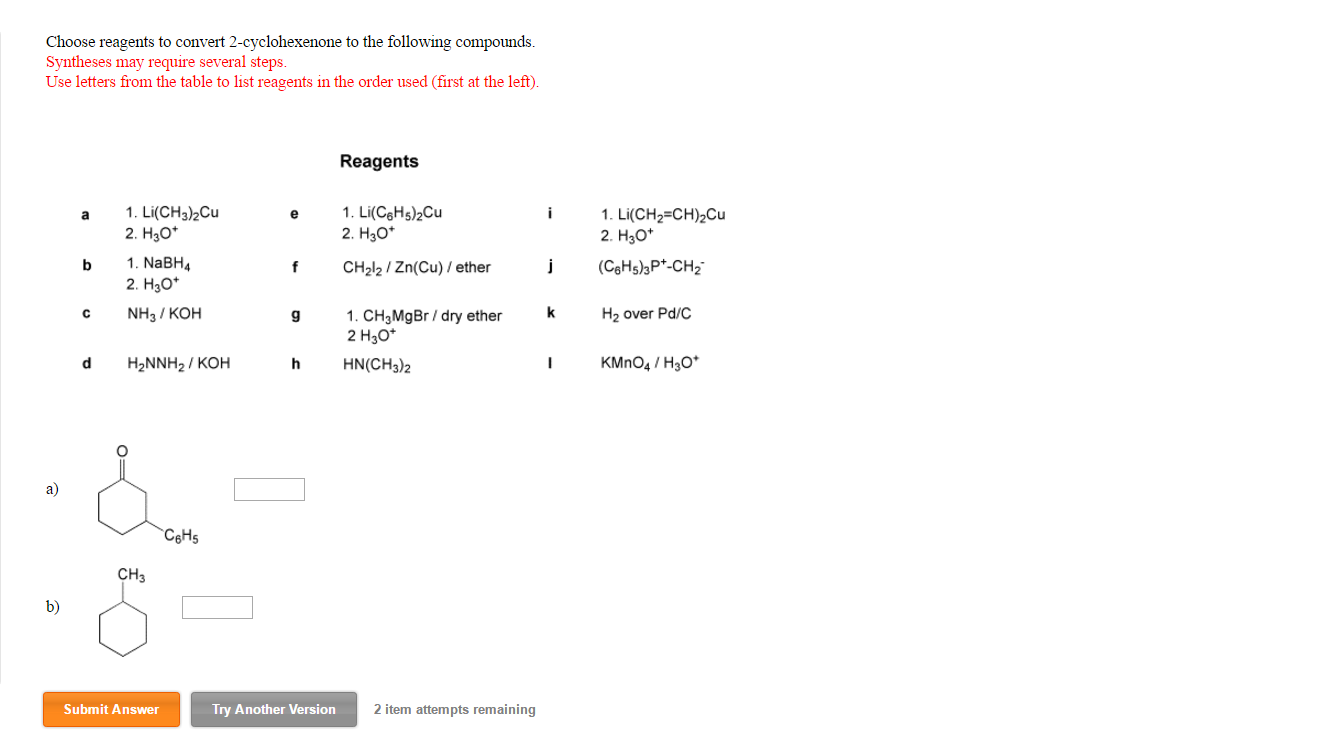
To convert 2-cyclohexenone to 2-cyclohexen-1-ol, we can employ a reducing agent. A very common and selective reagent for this type of transformation is sodium borohydride (NaBH₄). This reagent is a mild reducing agent and is particularly good at reducing ketones and aldehydes to alcohols without affecting other parts of the molecule, such as the double bond in our case. The sodium borohydride delivers a hydride ion (H⁻) to the carbonyl carbon, which then leads to the formation of the alcohol.
The benefit of this transformation? 2-cyclohexen-1-ol, with its hydroxyl group and double bond, is itself a versatile intermediate. It can be further modified to create a wide range of other organic compounds, finding applications in areas like fragrances, pharmaceuticals, and even in the synthesis of more complex natural products. It showcases how chemists can precisely alter specific functional groups to build molecular complexity step by step.
The Art of Chemical Design
These two examples, while simple, highlight the elegance and power of organic chemistry. By understanding the reactivity of molecules like 2-cyclohexenone and knowing the properties of different reagents, chemists can design pathways to create virtually any molecule imaginable. It’s a process of careful selection, precise execution, and creative problem-solving. The ability to convert one compound into another, like turning 2-cyclohexenone into cyclohexanone or 2-cyclohexen-1-ol, is fundamental to scientific progress and innovation across countless industries.
