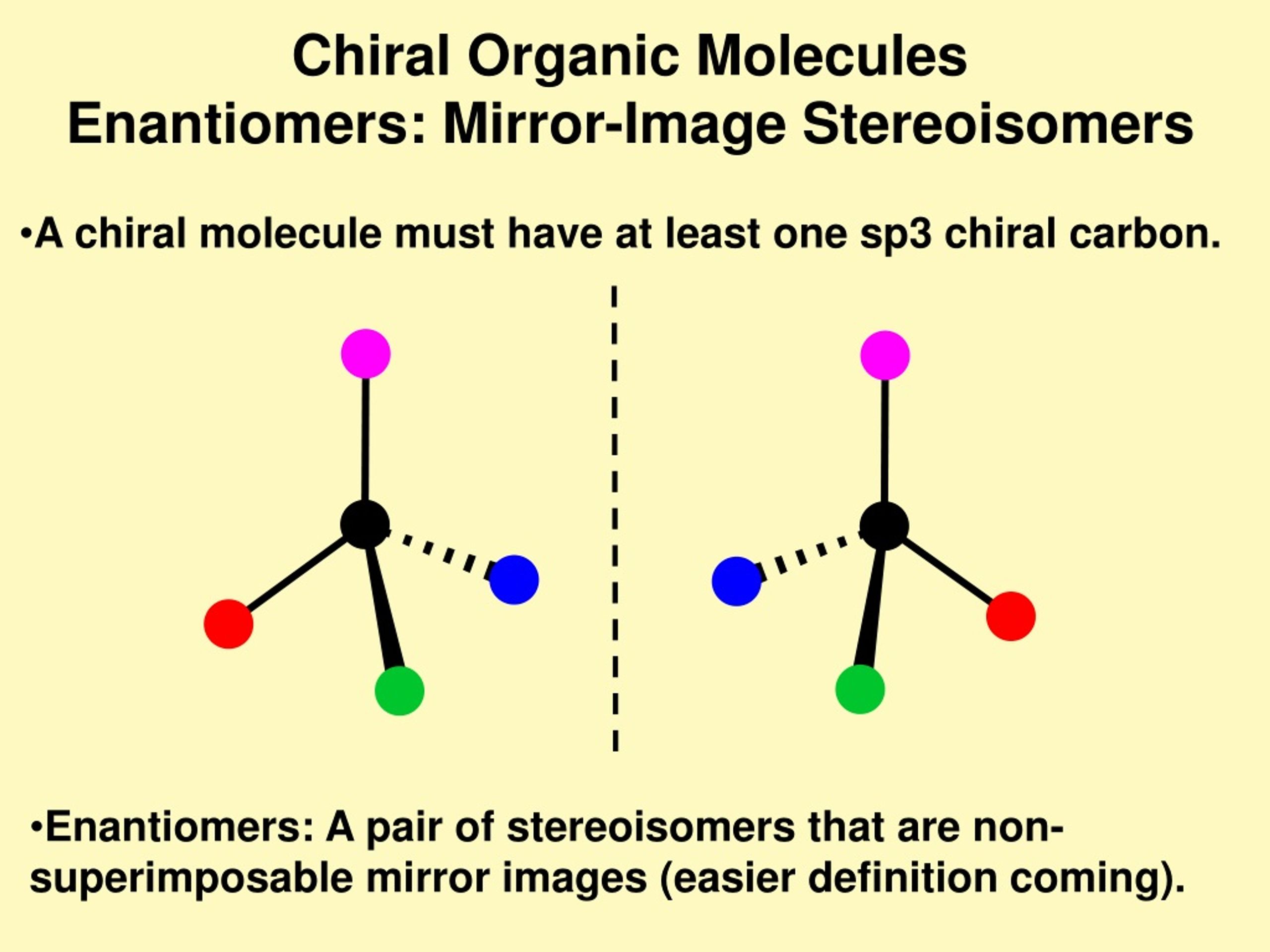
Chirality Occurs When Stereoisomers Have Mirror Images That Are
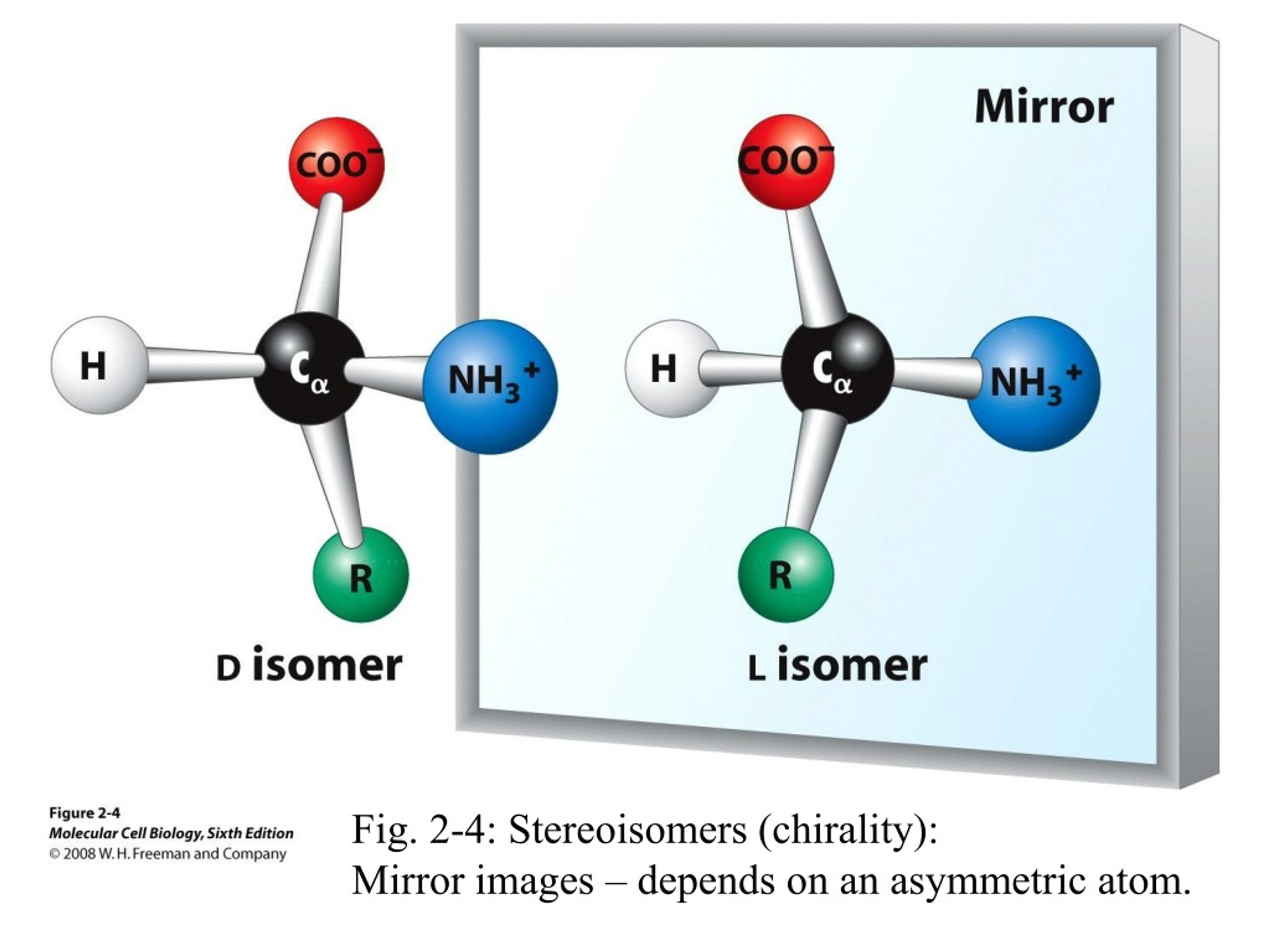
Hey there, you! Grab your mug, settle in. We're gonna chat about something super cool, something that’s kinda like a magic trick in the world of molecules. Ever looked in a mirror and thought, "Yep, that’s me, but… backwards"? Well, get ready, because that’s basically the vibe of chirality. It’s when molecules get a little… two-faced. Like a coin, but way more complex and, honestly, way more important for, you know, everything.
So, what's the big deal? Chirality happens when you have these things called stereoisomers. Think of them as identical twins, but with a tiny, but huge, difference. They have the same exact atoms, connected in the same exact order, but their 3D arrangements are different. It’s like having two identical LEGO sets, but one is built facing left and the other is built facing right. See? Same bricks, same connections, totally different orientation in space. Wild, right?
And when these stereoisomers are like mirror images of each other, and they can't be perfectly laid on top of each other? Boom! That’s chirality. It's like your left hand and your right hand. They're mirror images, totally. You can't, like, put your right glove on your left hand and expect it to fit perfectly, can you? Nope. They’re different. They’re chiral. Your hands are chiral. Mind. blown. I know, right? Who knew our own hands were such a textbook example of fundamental chemistry?
This whole mirror-image thing is a biggie. Imagine a molecule. It’s got these little arms (bonds) sticking out, holding onto other little things (atoms). Now, if you arrange those arms and atoms in a certain way, you can get a molecule. But then, if you flip it in a mirror… and that flipped version is not the same as the original, no matter how you twist and turn it… congratulations, you've got yourself a chiral molecule! It’s like a dance move. You can do the same steps, but in a mirrored way, and it just looks… different. Not wrong, just different.
Why should you care about molecules looking like they’re doing the mirror image dance? Oh, honey, because it’s everywhere. Seriously. Think about your body. Your DNA, your proteins, the way your enzymes work – all of it is incredibly sensitive to this chirality thing. It’s not just a neat little molecular quirk; it’s a fundamental principle that dictates how life itself functions. Pretty heavy, huh? For something that looks like a simple mirror image trick, it’s got some serious power.
Let's dive a little deeper, shall we? We call these non-superimposable mirror image molecules enantiomers. Fancy word, I know. But it just means "opposite" in Greek. So, you have a molecule, and then you have its enantiomer. They’re like the yin and yang of the molecular world. They have the same properties, like melting point and boiling point, unless… well, unless they interact with something else that's also chiral. And guess what? A lot of things in the universe are chiral. Like light! Or other molecules! This is where things get really interesting, and maybe a tiny bit confusing, but stick with me!
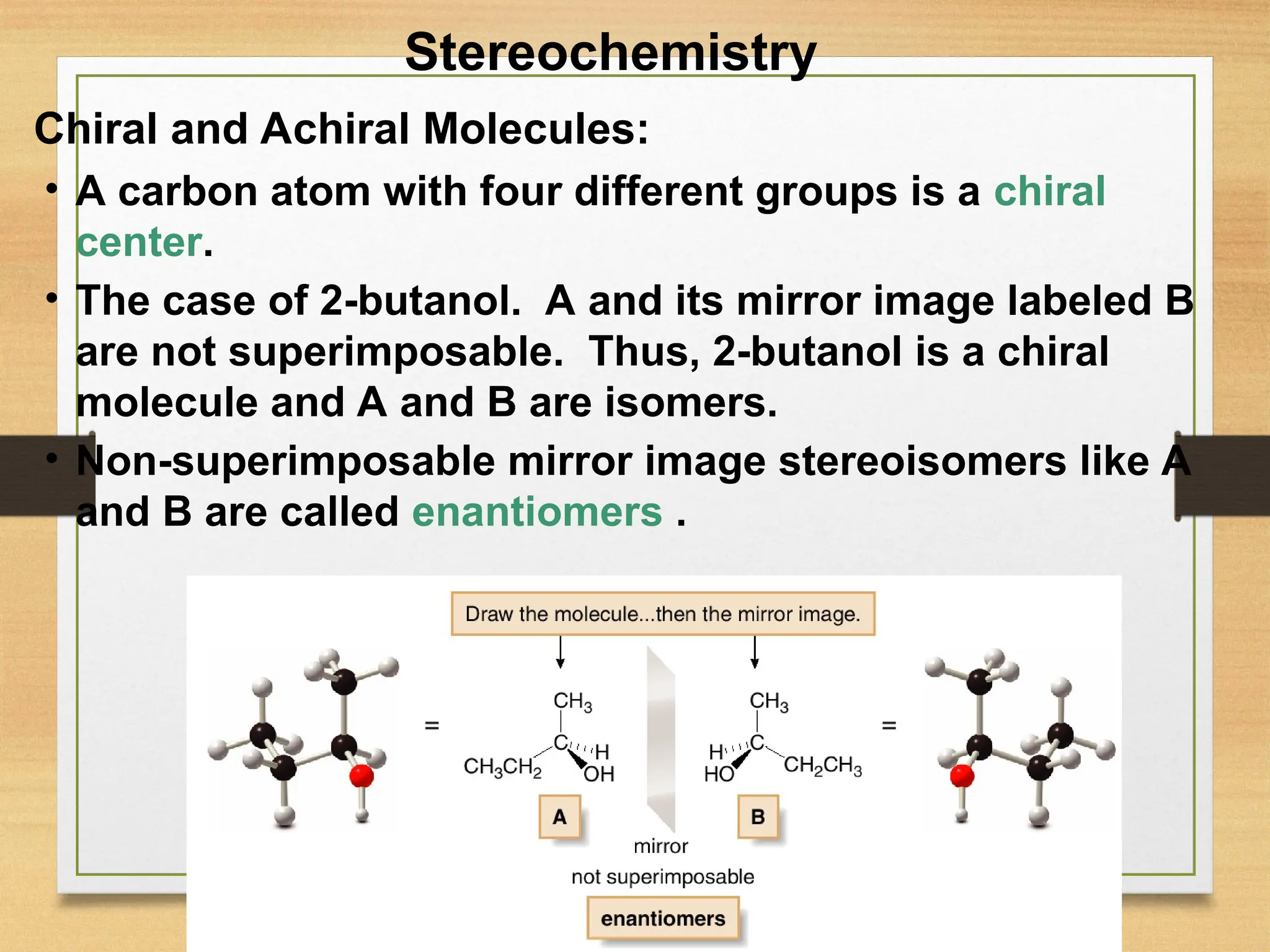
Imagine you’re making something, say, a drug. This drug is a molecule. Now, if this molecule is chiral, it can exist in two enantiomeric forms. Let's call them the "R" form and the "S" form (just for fun, don't worry about the actual R and S yet). These two forms, even though they look so similar, can have vastly different effects on your body. It’s like having two keys that look almost identical, but only one of them will unlock your front door. The other one? Might just jam the lock, or worse!
This is crucial in medicine. The thalidomide tragedy is a grim reminder of this. One enantiomer of thalidomide was great for morning sickness. The other enantiomer? Caused severe birth defects. Oof. Just… oof. It’s a stark example of how something as subtle as a 3D arrangement can have such profound and devastating consequences. It really makes you pause and think, doesn't it? Chemistry isn't just about reactions; it's about shapes and orientations and how they interact with the incredibly complex, chiral world of biology.
So, how do scientists tell these chiral molecules apart? They have special techniques, of course! They use something called optical activity. Remember how I said the enantiomers behave differently when interacting with other chiral things? Well, chiral molecules can rotate plane-polarized light. One enantiomer might rotate it clockwise, and the other will rotate it counterclockwise, by the exact same amount! It’s like they’re both dancing with polarized light, but doing opposite spins. Pretty neat, huh? This is how you can tell if you've got the "good" enantiomer or the "bad" one, or at least, the one that does what you want it to do.
Think about flavors and smells, too. That's another area where chirality is king. The reason one enantiomer of a molecule might smell like lemons and the other smells like pine needles? It’s because our noses are filled with chiral receptors! These receptors are shaped in a specific way, and only one of the enantiomers will fit perfectly into the receptor to trigger that smell. It's like trying to put your left hand into a glove designed for your right. It just doesn't quite feel right, does it? Or taste right, in this case.
Carvone is a classic example. One enantiomer tastes and smells like caraway seeds (think rye bread!). The other enantiomer? It’s the star of spearmint gum! Same atoms, same connections, just a different 3D twist. Isn’t that wild? You're chewing spearmint gum, and it's all thanks to a little molecular chirality. It's a testament to how the universe is built on these intricate, spatial relationships. Who knew chewing gum could be so scientifically significant?
And it’s not just about drugs and flavors. Think about agriculture. Certain pesticides are chiral. Only one enantiomer might be effective against a particular pest, while the other is just… useless, or worse, harmful to other organisms. So, chemists work hard to make sure they’re producing the right enantiomer, the one that does the job cleanly and efficiently. It’s all about precision and understanding these subtle molecular differences.
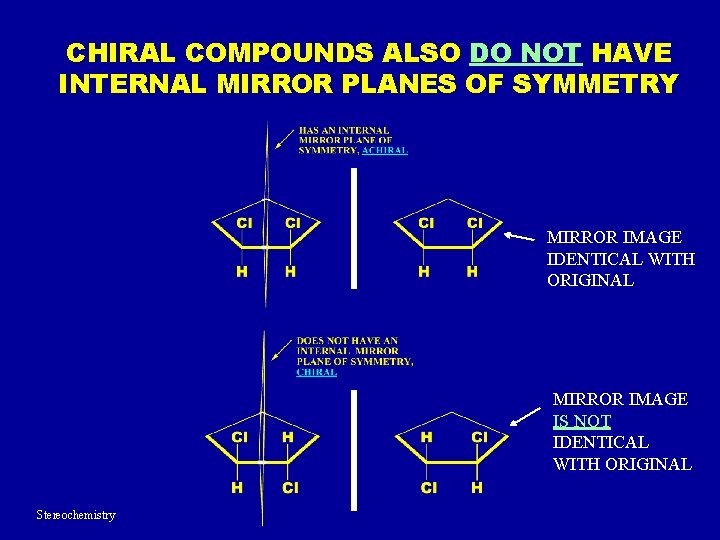
Now, not all molecules are chiral, obviously. Some are just… plain. They have a plane of symmetry, which is like a mirror running through the middle of the molecule. If you can slice a molecule in half, and one half is a perfect mirror image of the other, then it’s not chiral. It’s like a perfect circle; it has infinite planes of symmetry. Or a square. You can cut a square in half both ways and get mirror images. These molecules are called achiral. They’re the boring, symmetrical ones of the molecular world. No mirror-image drama for them!
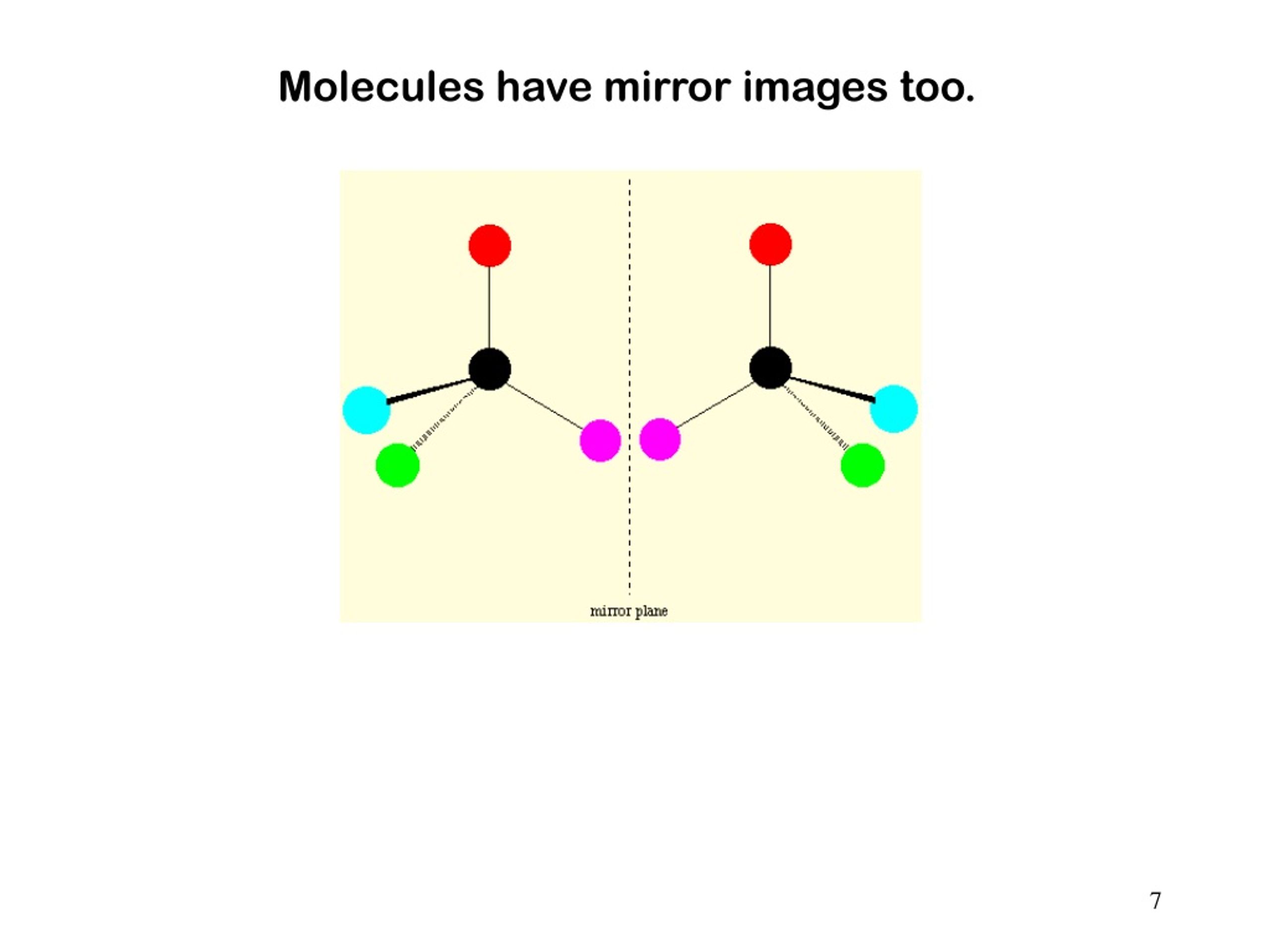
But the ones that don't have that symmetry, the ones that are a bit lopsided in their 3D arrangement? Those are the ones that can be chiral. They usually have a special atom called a chiral center. This is typically a carbon atom bonded to four different groups. Think of it like a hub with four spokes, and each spoke is a different color. If you try to arrange those colors in a mirror image, you’ll see they’re different. You can’t make them overlap perfectly. It’s the classic way to identify potential chirality in many organic molecules. That little carbon atom is the source of all the hand-waving and mirror-image fuss.
So, when you’re looking at a molecule and trying to figure out if it’s chiral, you’re often looking for that carbon with four different things attached. If you find one, there’s a good chance you’ve got a chiral center, and therefore, a chiral molecule! It’s like a molecular treasure hunt. And once you find it, you know it has a mirror-image twin that’s not quite the same.
The process of creating just one specific enantiomer is called asymmetric synthesis. It's like being a molecular sculptor, carefully crafting the molecule into the exact shape you want, rather than just making a random mix. This is super important in the pharmaceutical industry because, as we saw with thalidomide, the difference can be life and death. They can’t just throw a bunch of molecules together and hope for the best. They need control. They need precision. They need to ensure they’re making the desired enantiomer, and only that one.
And sometimes, even nature gets it wrong! Well, not wrong, but it can produce a mixture of both enantiomers. This mixture is called a racemic mixture, or a racemate. It's like having a 50/50 split of your left and right hands. In many biological processes, however, enzymes are highly specific and will only work with one enantiomer. So, even if you have a racemic mixture, the body might only "see" and use half of it, leaving the other half as, well, just extra baggage.
It’s a bit like trying to fit a square peg in a round hole, but with molecules. Our bodies are full of chiral structures – proteins, sugars, DNA – and they are incredibly picky about which enantiomer they interact with. It’s not just about having the right parts; it's about having them in the right orientation. This is why chirality is so fundamental to biochemistry. It’s the basis for molecular recognition, for enzymes binding to their substrates, for how our senses perceive the world.
Think about it from an evolutionary perspective. Life on Earth, for the most part, is homochiral. That means most of our biological molecules tend to be one specific enantiomer. For instance, amino acids in proteins are almost exclusively the "L" form, and sugars in DNA and RNA are the "D" form. Why? Nobody’s entirely sure, but it’s a deeply ingrained feature of life. It’s like the universe chose a particular hand to build life with, and it’s stuck with it. Maybe it was a cosmic coin flip that determined left or right orientation for life’s building blocks, and that’s just how it played out!
This homochirality has implications for astrobiology too. If we ever find life on another planet, will it also be homochiral? Will it use the same "handedness" of molecules, or will it have its own, entirely different chiral preference? That's one of the huge questions scientists are exploring. Discovering alien life that uses the opposite enantiomers would be absolutely mind-boggling. It would prove that the chirality we see on Earth isn’t necessarily the only way life can be built.
So, next time you look at your hands, or smell a flower, or even just take a sip of your coffee, remember this little chat about chirality. Those seemingly simple molecules are masters of 3D arrangement, and their mirror-image duality is a cornerstone of our existence. It’s a reminder that even the smallest, subtlest differences can have the biggest impacts. It’s like the universe’s way of saying, "Hey, it’s not just what you are, but how you're shaped that really matters!" Pretty cool, right? Now, go forth and ponder the chiral wonders of the world!
