Chemistry Unit 7 Chemical Reactions Rearranging Atoms Answer Key

You know, I was rummaging through an old box the other day, one of those dusty forgotten corners of the attic, and I found a bunch of my old high school notebooks. Seriously, it was like a time capsule. I stumbled upon my Chemistry Unit 7 notes, specifically the section on Chemical Reactions and Rearranging Atoms. And let me tell you, seeing that "Answer Key" scribbled on the corner of a page gave me a moment of pure, unadulterated nostalgia. Remember that feeling? That mix of dread and triumphant relief when you finally saw the right answers after staring at a problem for what felt like an eternity?
It got me thinking, though. This whole "rearranging atoms" thing, it’s such a fundamental concept, isn't it? We learn it in high school, we might even ace a quiz on it (thanks, answer key!), but do we really get what it means? It’s not just about balancing equations; it’s about the very fabric of our universe. So, let's dive back into that glorious world of Unit 7, shall we? Forget the dry textbook for a moment and let’s chat about what’s really going on when atoms decide to, you know, move in.
The Great Atom Shuffle: It's Not Magic, It's Chemistry!
So, picture this: you’re making a cake. You’ve got your flour, your sugar, your eggs, your butter. All these ingredients are made of atoms, right? Carbon, hydrogen, oxygen, nitrogen – the usual suspects. When you mix them all together and bake them, something pretty amazing happens. The flour doesn't just sit there, nor does the sugar. They don't magically fuse together. Nope. What’s actually happening is a chemical reaction. The atoms within those ingredients are getting a serious makeover. They’re breaking old bonds and forming new ones. The result? A delicious cake, a completely different substance from its individual components. Pretty wild when you think about it, huh?
This is the core of Chemistry Unit 7. Chemical reactions are all about rearranging atoms. It’s like a cosmic game of LEGOs, but with incredibly tiny, invisible bricks. You start with certain building blocks (reactants), and through the magic of chemical energy, they get dismantled and reassembled into completely new structures (products).
And that's where that elusive "Answer Key" comes into play, right? Because understanding these rearrangements, especially when you’re just starting out, can feel like trying to solve a Rubik's Cube blindfolded. You see the before, you see the after, but how did it get there? The answer key, bless its little page-numbered heart, was our guide, our sanity check.
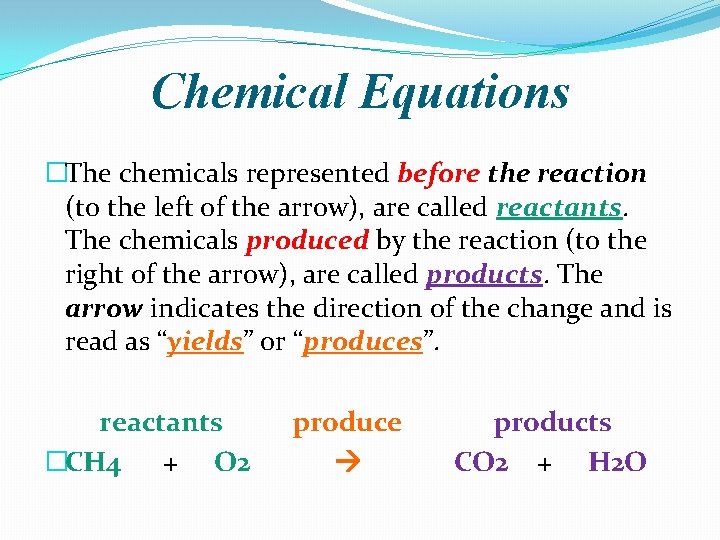
From Ingredients to Equations: The Language of Change
Let’s get a bit more technical for a sec, but I promise, no pop quizzes! The way chemists talk about these atom rearrangements is through something called a chemical equation. Think of it as a shorthand for the entire process. You’ve got your reactants on the left side, the things that are going into the reaction. Then you have an arrow (that’s the important "yields" or "produces" part), and on the right side, you’ve got your products, the new things that have been created.
A classic example, and one you probably encountered in Unit 7, is the formation of water. You take hydrogen gas (H₂) and oxygen gas (O₂), and poof (well, with a little spark or heat), you get water (H₂O). The equation looks something like this:
2H₂ + O₂ → 2H₂O
See those numbers? Those aren't just for decoration. They’re crucial! They tell us about the conservation of mass. In any chemical reaction, atoms aren't created or destroyed; they're just shuffled around. So, if you start with four hydrogen atoms (two H₂ molecules), you need to end up with four hydrogen atoms in your products. And if you start with two oxygen atoms (one O₂ molecule), you need to end up with two oxygen atoms.
This is where the "balancing" part of chemistry comes in. It's like making sure you have exactly the right number of LEGO bricks to build your new creation, no more, no less. And honestly, that's where I remember spending a good chunk of my Unit 7 time – staring at those little numbers, trying to make both sides of the equation match up. The answer key was my lifeline.
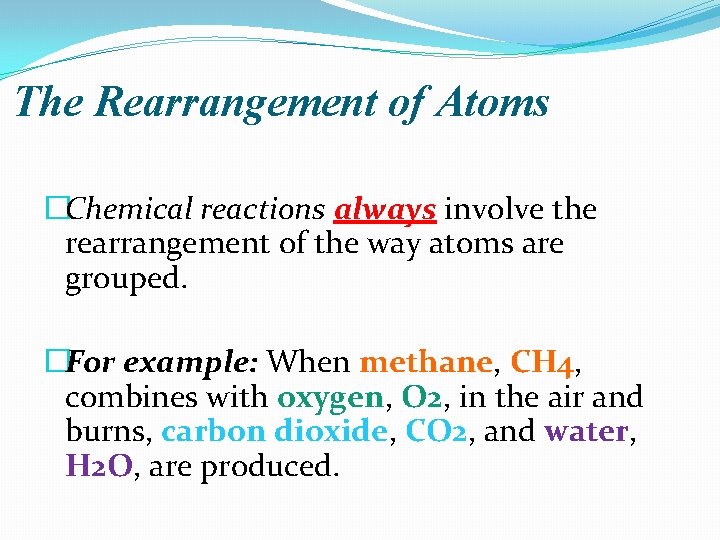
Why Do Atoms Bother Rearranging Anyway?
So, if atoms are just chilling, minding their own business in their existing molecules, why do they decide to break up and find new partners? Great question! It all comes down to energy and stability. Think of it like relationships. Sometimes, people are in relationships that aren't making them happy, or they see a better opportunity for fulfillment elsewhere. Atoms are kind of similar.
Atoms like to be in the most stable state possible. This usually means having a full outer electron shell. When atoms are bonded together, they're sharing or transferring electrons to achieve this stability. But sometimes, the energy required to break those existing bonds is less than the energy released when new, more stable bonds are formed. That's when a reaction is likely to happen.
Some reactions release energy (these are called exothermic reactions). Think of burning wood. That’s a lot of heat and light being given off. Other reactions require energy to happen (these are endothermic reactions). Photosynthesis is a classic example – plants need sunlight (energy!) to turn carbon dioxide and water into glucose and oxygen. It's all a big energy game.
It's not just random chaos; it's a drive towards a lower energy state, a more comfortable arrangement for those little guys. And understanding this energy exchange is a huge part of chemistry. It explains everything from why your hands feel cold when you use an instant ice pack to why engines run.
The Types of Rearrangements: Not All Shuffles Are Created Equal
Chemistry teachers, bless their organized hearts, love to categorize things. And chemical reactions are no exception. Unit 7 likely introduced you to a few main types of these atom rearrangements. Let’s recap, because it’s good to have these mental bookmarks.
We have:
- Synthesis Reactions: This is where two or more simple substances combine to form a more complex one. Think A + B → AB. It’s like building something up from smaller parts. Like when hydrogen and oxygen combine to make water.
- Decomposition Reactions: This is the opposite of synthesis. A complex substance breaks down into simpler ones. Think AB → A + B. Like how water can be broken down into hydrogen and oxygen (with enough energy, of course!).
- Single Displacement (or Single Replacement) Reactions: Here, one element replaces another in a compound. Think A + BC → AC + B. It’s like one partner in a dance twirling off with someone else. For example, if you have zinc metal reacting with hydrochloric acid, the zinc replaces the hydrogen in the acid.
- Double Displacement (or Double Replacement) Reactions: This is where the positive and negative ions of two ionic compounds essentially swap partners. Think AB + CD → AD + CB. It’s a bit of a dance-off where everyone changes partners. Often, this results in the formation of a precipitate (a solid that sinks to the bottom), a gas, or water.
- Combustion Reactions: These are reactions involving a fuel (usually a hydrocarbon) reacting rapidly with an oxidant, usually oxygen, to produce heat and light. Think of burning anything, really! They typically produce carbon dioxide and water.
Learning these categories was essential for predicting what would happen when you mixed different chemicals. And again, the answer key was there to confirm if your prediction, based on the type of reaction, was actually correct. It’s a bit like learning the rules of a new game; once you know the rules, you can start playing strategically.
Beyond the Textbook: Where We See Rearranging Atoms Every Day
It’s easy to think of chemistry as something that happens in sterile labs with beakers and Bunsen burners. But honestly, chemical reactions are happening all around us, all the time. Unit 7 wasn't just about abstract concepts; it was a gateway to understanding the world in a deeper way.
Think about it:

- Digestion: When you eat food, your body is performing a complex series of chemical reactions to break down that food into usable energy and nutrients. Your stomach acid (hydrochloric acid) is a key player here!
- Rusting: That lovely orange-brown stuff on an old bike? That's iron reacting with oxygen and water to form iron oxide. A classic example of a single displacement reaction (though a bit slower than the lab version!).
- Breathing: The exchange of oxygen and carbon dioxide in your lungs is a vital chemical process. Hemoglobin in your blood binds to oxygen, and then releases it to your tissues, picking up carbon dioxide in return.
- Cooking: As we mentioned with the cake, cooking is essentially a massive controlled chemical reaction. Browning meat, making toast, even boiling an egg – all involve atoms rearranging.
- Batteries: That phone in your hand? It works because of carefully controlled chemical reactions happening inside its battery, converting chemical energy into electrical energy.
Every time you strike a match, every time a plant grows, every time you feel the warmth of the sun (that’s nuclear fusion, but the principles of energy release are related!), you're witnessing the power of atoms rearranging.
The Answer Key's Legacy: More Than Just Right Answers
Looking back at that old Unit 7 answer key, it’s funny. It represents a time of learning, of struggle, and ultimately, of understanding. It wasn’t just about getting the "right" answer to a specific question. It was about internalizing the why and the how behind those rearrangements.
It taught us that matter isn't static. It's in a constant state of flux, transforming and evolving. It’s a humbling thought, really. We're made of the same stuff as stars, and that stuff is constantly dancing, bonding, and rebonding.
So, the next time you see something change – a fire burning, food cooking, even just rust forming on a nail – take a moment. Remember Unit 7. Remember the rearranging atoms. Because it’s not just chemistry; it’s the fundamental story of how our universe works. And that, my friends, is pretty darn cool. Maybe you’ll even start seeing the world a little differently, with a bit more appreciation for all those invisible, energetic dances happening all around you. Now, if you’ll excuse me, I think I need to go bake something. For science, of course.
