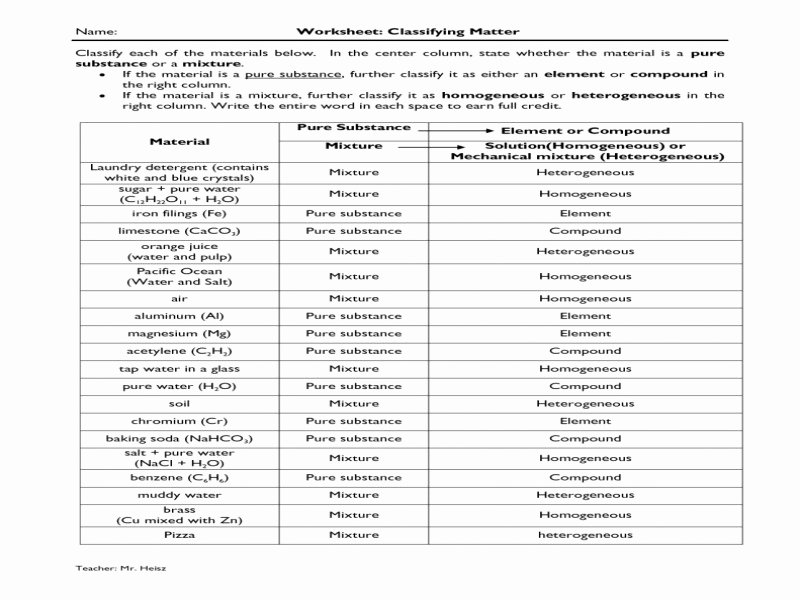
Chemistry 1 Worksheet Classification Of Matter And Changes Answer Key

Alright, settle in, grab your virtual latte, and let's talk about something that sounds like it belongs in a dusty textbook but is actually as exciting as discovering you have a secret superpower... well, almost. We're diving headfirst into the thrilling world of chemistry worksheets, specifically the ones about
Now, I know what you're thinking. "Chemistry worksheet? Sounds like my worst nightmare has returned to haunt my coffee break." But hear me out! This isn't your old high school chemistry class where Mrs. Crabtree made you memorize the atomic weight of Osmium (which, by the way, is 190.23. You're welcome. That's a useless fact you can now deploy at parties). This is about understanding the very stuff that makes up… well, everything.
Imagine you're at a buffet. A cosmic buffet, if you will. You've got all sorts of delicious things laid out: fluffy mashed potatoes, a towering stack of pancakes, maybe some questionable-looking Jell-O. Chemistry is basically the science of figuring out what those things are made of, and how they got that way. And our worksheet? It’s the little napkin you get to jot down your observations. The answer key? That’s when the waiter comes over and tells you, "Yep, those are indeed pancakes, and they were made with flour, eggs, and a dash of pure awesome."
So, What's This "Matter" Thing Anyway?
Matter, my friends, is anything that has
Now, chemistry doesn't just stop at "Yep, it's stuff." Oh no. It gets fancy. It starts classifying this stuff. It’s like sorting your sock drawer, but with way more dramatic consequences if you get it wrong. We’re talking about

Pure Substances: The Solo Artists of the Universe
Pure substances are the divas of the matter world. They're either
Compounds are like supergroups. They’re formed when two or more elements join forces, chemically bonded. Water (H₂O) is a classic example. It’s made of hydrogen and oxygen, but it’s not just a mixed bag. It’s a brand-new entity with completely different properties. Imagine if your grumpy Uncle Steve and your super-enthusiastic Aunt Carol got married and suddenly became a serene meditation guru. That’s a compound! They’re still Uncle Steve and Aunt Carol underneath, but their combined effect is something entirely new.
Mixtures: The Potlucks of Matter
Then we have mixtures. These are the parties where everyone’s invited, and you can still pick out the individual guests. Mixtures are just physical combinations of pure substances. They haven’t gone through the dramatic marriage ceremony of chemical bonding. You can usually separate them with a bit of elbow grease or some clever trickery. Think of a salad. You can still see the lettuce, the tomatoes, the croutons, right? That’s a
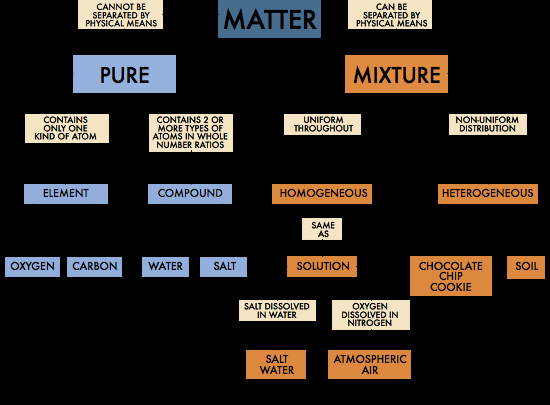
On the flip side, you have
The Plot Twist: Changes!
Now, for the really juicy part:
Physical changes are like rearranging the furniture in your house. You’re changing its appearance or state, but the fundamental “you” remains the same. Melting ice? It’s still water, just in a different form. Boiling water? Still water, just having a really, really energetic party. Breaking a glass? It’s still glass, just in smaller, more dangerous pieces. The chemical composition hasn't changed. It's like putting on a fancy hat; you're still the same person, just looking a bit more festive.
Chemical Changes: The Dramatic Transformations
Chemical changes, however, are the plot twists that make you spill your coffee. This is where the actual transformation happens, where new substances are formed with entirely new properties. Burning wood? That’s a chemical change. You end up with ash, smoke, and gases, which are not wood anymore. Rusting iron? Also a chemical change. You've gone from shiny metal to flaky, reddish-brown gunk. It's like your car turning into a sentient, grumpy robot that only communicates in squeaks and rattles. That's a chemical change of epic proportions!
How do you tell the difference? Well, sometimes it’s obvious. If you see fire, hear explosions, or notice a dramatic color change, it’s probably chemical. Other times, you might look for signs like the production of gas (bubbles!), a change in temperature (gets hot or cold!), or the formation of a precipitate (a solid forming from a liquid solution). It’s like detective work for atoms!

The Elusive Answer Key
So, where does this mythical
It’s the scientific equivalent of a gold star, a virtual pat on the back, or the satisfying click when a puzzle piece finally fits. It confirms your understanding and, more importantly, helps you learn from your mistakes. Because, let's be honest, even the greatest scientists make mistakes. Newton probably dropped a few apples on his head before he figured out gravity, and Marie Curie likely had a few minor lab mishaps before revolutionizing radioactivity. The key is to learn and keep experimenting (safely, of course!).
So, next time you encounter a chemistry worksheet, don't groan. See it as an invitation to explore the fascinating, ever-changing, and surprisingly delicious world around you. And if you happen to find that answer key, well, consider it your passport to a deeper understanding. Now, if you'll excuse me, I think I saw some questionable Jell-O on that cosmic buffet, and I'm suddenly very curious about its classification.
