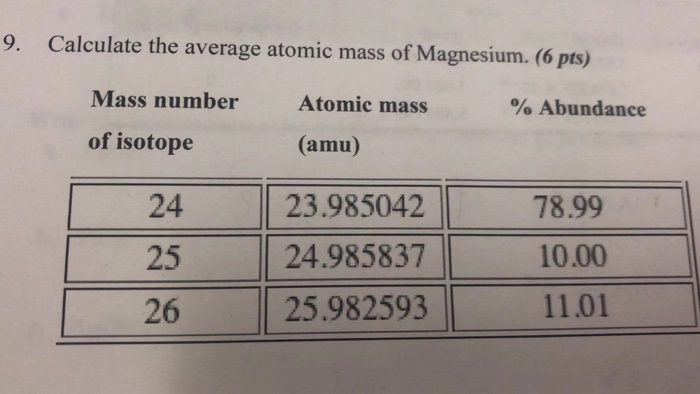
Calculate The Average Atomic Mass Of Magnesium
Ever wonder about the invisible universe that makes up everything around us? It’s like a giant, cosmic Lego set where each tiny piece, an atom, has its own special flavor. Today, we’re going to peek at the flavor profile of one of these atoms – a metal we all know and love, even if we don’t always think about it.
Yep, we’re talking about Magnesium! Think about those fizzy tablets you might pop when you’re feeling a bit under the weather, or the lightweight frames of your bicycle. All that owes a little something to our friend Magnesium.
But here’s where it gets interesting, like a recipe with a surprise ingredient. Not all Magnesium atoms are exactly the same. Imagine a baker making cookies. They might use slightly different sizes of chocolate chips, but they're all still chocolate chips, right?
Well, Magnesium atoms can be a little bit like that. Most of them are pretty similar, but some have a few extra tiny particles inside them. These extra bits don't change what makes a Magnesium atom a Magnesium atom, but they do make it a smidge heavier.
These slightly different versions of the same atom are called isotopes. It's like having different flavors of ice cream – all ice cream, but with a twist! For Magnesium, we have a few common flavors.
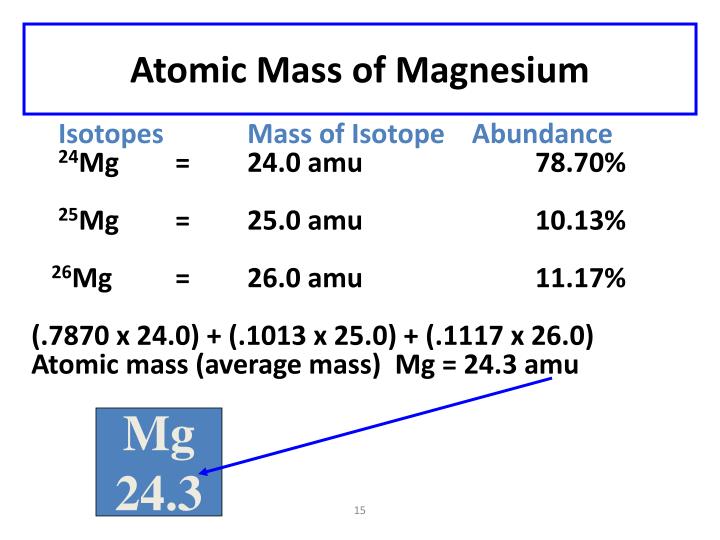
The most common flavor, the vanilla of Magnesium isotopes, is called Magnesium-24. This is the one you’ll find most often, just hanging out in the universe. It’s the reliable friend who’s always there.
Then we have Magnesium-25. This one’s a bit like the mint chocolate chip version – still clearly ice cream, but with a subtle difference. It's a little less common than Magnesium-24.

And finally, there’s Magnesium-26. This is the surprise swirl, maybe a raspberry ripple in your vanilla. It’s the least common of the three main Magnesium flavors.
Now, scientists, bless their curious hearts, like to know the average weight of these atoms. It’s not just about one specific atom, but what you'd expect to find on average if you grabbed a handful of Magnesium. This average weight is what we call the average atomic mass.
Think of it like trying to figure out the average height of your friends. You wouldn’t just measure the tallest person or the shortest; you’d get everyone’s height and then figure out the middle ground.
So, how do we find the average atomic mass of Magnesium? It’s not as complicated as it sounds. We just need to know two things: what are the different flavors (isotopes) and how common is each flavor?
Scientists have done all the hard work of figuring out the exact weight of each Magnesium isotope. They're like super-powered scales that can weigh individual atoms! And they've also figured out the percentage of each isotope that exists naturally. It's like knowing that 80% of your cookies have regular chocolate chips, 15% have larger ones, and 5% have extra-dark ones.
For Magnesium-24, it’s the rockstar. It makes up about 79% of all the Magnesium out there. That’s a huge chunk!
Magnesium-25 is a bit of a supporting actor, showing up about 10% of the time. Still important, but not the headliner.
And Magnesium-26, our surprise flavor, is present in about 11% of all Magnesium. It adds a little extra something to the mix.
Now, to get the average, we do a little bit of math. It’s like calculating your average grade in school. You take the value of each item (the weight of the isotope), multiply it by how often it appears (its abundance or percentage), and then add all those results together.
So, for Magnesium, we take the weight of Magnesium-24 and multiply it by its percentage. Then, we do the same for Magnesium-25 and Magnesium-26.

We’re essentially saying, "Okay, most of the time, we're dealing with this weight (Magnesium-24), sometimes with this other weight (Magnesium-25), and a little bit with this third weight (Magnesium-26)."
When you add all these weighted amounts together, you get a single number. This number is the average atomic mass of Magnesium. It’s the most representative weight for a Magnesium atom, even though no single atom might be exactly that weight.
The number you’ll usually see for the average atomic mass of Magnesium is around 24.305. That’s not a whole number, and that’s okay! It's a testament to those different isotopes.
It’s like saying the average height of your class is 5 feet 7.3 inches. Most kids might be a little taller or a little shorter, but that’s the overall average.
Why is this important, you ask? Well, it’s crucial for scientists who work with elements. When they’re designing new materials, understanding chemical reactions, or even figuring out how old things are, they need to know these average masses.

It's the foundation for so much of what we understand about the physical world. Without knowing these average weights, our understanding of chemistry would be like trying to build with Legos without knowing how many studs each piece has.
So, the next time you see something made of Magnesium, whether it's a firework bursting with bright light or a piece of chalk used in gymnastics, remember its little atomic secret. It's a blend of slightly different, but equally important, versions of itself.
And that average atomic mass? It’s a beautiful reminder that even in the tiniest building blocks of the universe, there’s diversity, and that diversity is what creates the wonderfully complex and interesting world we live in. It’s a small number that tells a big, fascinating story.
Isn't it amazing that something as fundamental as an atom's weight is a kind of average, a consensus from its slightly varied relatives? It's like the universe giving each element its own unique, subtle personality!
So, go ahead, give a little nod to Magnesium and its isotopes. They’re working hard, making everything from your bones stronger to your camera lighter, all while contributing to that wonderful average atomic mass number.
It's a fun little peek behind the curtain of the atomic world, proving that even the most basic science can have a touch of wonder and a whole lot of everyday magic.
