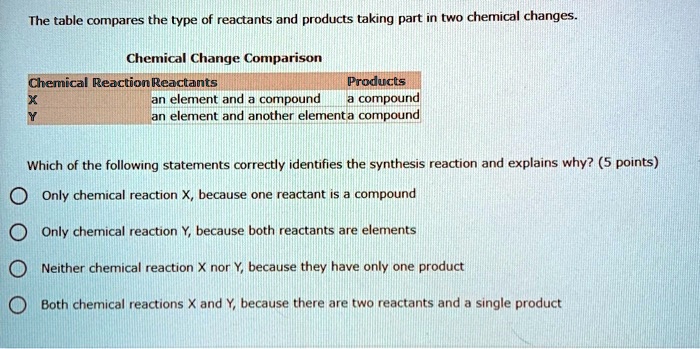
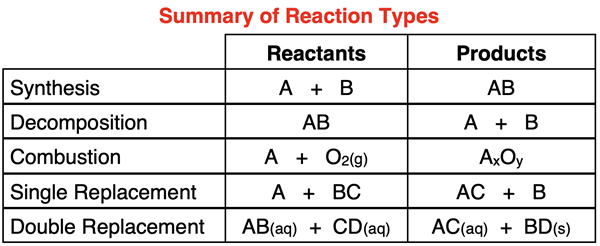
Based On Reference Table J Which Two Reactants React Spontaneously

Imagine a world where certain pairings just click. They meet, and bam! Instant reaction, a spark, a guaranteed good time. It's like a cosmic matchmaking service for molecules, and today, we're peeking behind the curtain to see which ones are the ultimate "meant to be" couples.
You know how some people just gravitate towards each other? They finish each other's sentences, have the same weird sense of humor, and generally make life brighter just by being around. Well, the same kind of magic happens in the tiny, unseen world of chemistry. We’re not talking about the dramatic explosions you see in movies (though sometimes those happen too!). We’re talking about a gentle nudge, a natural inclination, a sigh of relief when two specific chemical buddies get together.
Our trusty guide in this adventure is a cool chart called Reference Table J. Think of it as a celebrity dating profile for elements and compounds. It lists them out, sort of like their "must-haves" and "deal-breakers" when it comes to forming new connections. It’s all about who’s willing to give up a little something to make a bigger something happen.
So, who are the undisputed champions of spontaneous romance, according to Table J? Prepare to be surprised by the sheer enthusiasm of some of these pairings. They’re not playing hard to get; they’re ready to mingle, and they do it with gusto!
Let’s spill the tea on the dynamic duo that steals the show: Fluorine and Lithium. Yep, you heard that right! These two are practically made for each other. It’s like the popular kid and the energetic puppy meeting at a party – instant best friends, no questions asked.
Why are they so enthusiastic? Well, Fluorine is like that super eager friend who’s always ready for the next big thing. It’s incredibly good at grabbing onto other things, almost desperately wanting to share its electrons. On the other hand, Lithium is a bit more laid-back, happy to let go of one of its own little buddies (an electron, in this case) if it means making a stable, happy new arrangement.
When Fluorine and Lithium get together, it’s a match made in chemical heaven. Fluorine is like, "Gimme, gimme, gimme!" and Lithium is like, "Here, take it, I’m happy to share!" And just like that, they form a bond, a solid partnership that’s practically unbreakable. It's a heartwarming tale of giving and receiving, all happening at the atomic level.
Think of it like this: you have a friend who absolutely loves to collect trading cards, and another friend who has a duplicate card they’re just itching to trade. It’s a win-win situation! They both leave the interaction happier and more complete. Fluorine and Lithium are the ultimate trading card enthusiasts of the chemical world.
But the fun doesn’t stop there! Table J reveals other incredibly eager pairings. It's not just a one-hit wonder; there's a whole social scene of molecules lining up to react. It’s like a speed-dating event where everyone’s got a smile and is ready to find their perfect match.
Another pair that gives a big thumbs-up on spontaneity is Fluorine and Potassium. Again, our superstar Fluorine is leading the charge. It's just that good at making connections.

Potassium, much like Lithium, is also quite happy to let go of an electron. It’s a fellow traveler on the "happy to share" path. So, when Fluorine comes knocking, Potassium opens the door with a big, welcoming smile. They create a strong, stable bond, and everyone’s happy.
It’s a bit like a popular influencer meeting an equally popular blogger. They see each other, recognize their shared interests (or in this case, their complementary electron needs), and poof! A collaboration is born. Their combined influence creates something even greater than they could have on their own.
You might notice a pattern here. Fluorine seems to be a bit of a social butterfly, connecting with a whole range of other elements. It’s the life of the chemical party, always initiating the fun.
The key to understanding these spontaneous reactions is looking at their position on Reference Table J. Elements at the very top are the most eager to react, like they’ve got an endless supply of energy and enthusiasm. The elements at the bottom? They’re more reserved, perhaps a little shy, and need a good reason to get involved.
So, when you see Fluorine, which is way up there at the top, meeting something like Lithium or Potassium, which are also relatively high up, it's a recipe for instant chemistry. It's like two people who are both super outgoing and just clicked from the moment they met. No awkward silences, just immediate connection and a shared excitement for what’s next.
It’s also interesting to see who doesn't react spontaneously. Imagine two people who are both perfectly happy being single and have no desire to change their status. They might be perfectly nice to each other, but there's no real "spark" that drives them to form a lasting partnership.
For example, if you tried to get something like Helium to react with something else on the table, it would likely be a very unenthusiastic encounter. Helium is like the contented hermit of the chemical world, perfectly happy in its own little space and not looking for any drama.
The beauty of Reference Table J is that it takes the guesswork out of it. It’s not about feelings or intuition; it's about the inherent properties of these substances. It’s a scientific love language that tells us which pairs are just destined to be.

So, next time you hear about chemical reactions, remember that they’re not all complicated and scary. Sometimes, they’re just two happy molecules finding each other and deciding to start a beautiful, stable relationship. It’s a reminder that even in the smallest corners of our universe, connection and spontaneity can lead to something wonderful.
It’s a charming thought, isn't it? That amidst all the complexities of science, there are these simple, undeniable urges to connect. Fluorine and Lithium, the original power couple. Fluorine and Potassium, the dynamic duo. They’re out there, making chemical magic happen, one spontaneous reaction at a time. And all it takes is a peek at a chart to see their love story unfold.
So, the next time you’re feeling a bit uncertain about a chemical interaction, just remember our super-eager friends. They’re the ones who prove that sometimes, the best things in life happen when you’re just ready to say "yes" to a new connection. It’s a heartwarming, and frankly, quite fun, way to look at the world around us.
And who knows? Maybe there's a little bit of Fluorine in all of us, always ready for the next exciting encounter. We’re all just looking for our perfect chemical match, after all!
