Balancing Chemical Equations With Polyatomic Ions Worksheet Answers
Ah, the thrilling world of balancing chemical equations! It’s like a secret spy mission, but with more numbers and less explosions. Except, you know, sometimes in real life, those numbers do lead to explosions. But for now, we’re just talking about worksheets. And maybe, just maybe, we have a little secret about those worksheets.
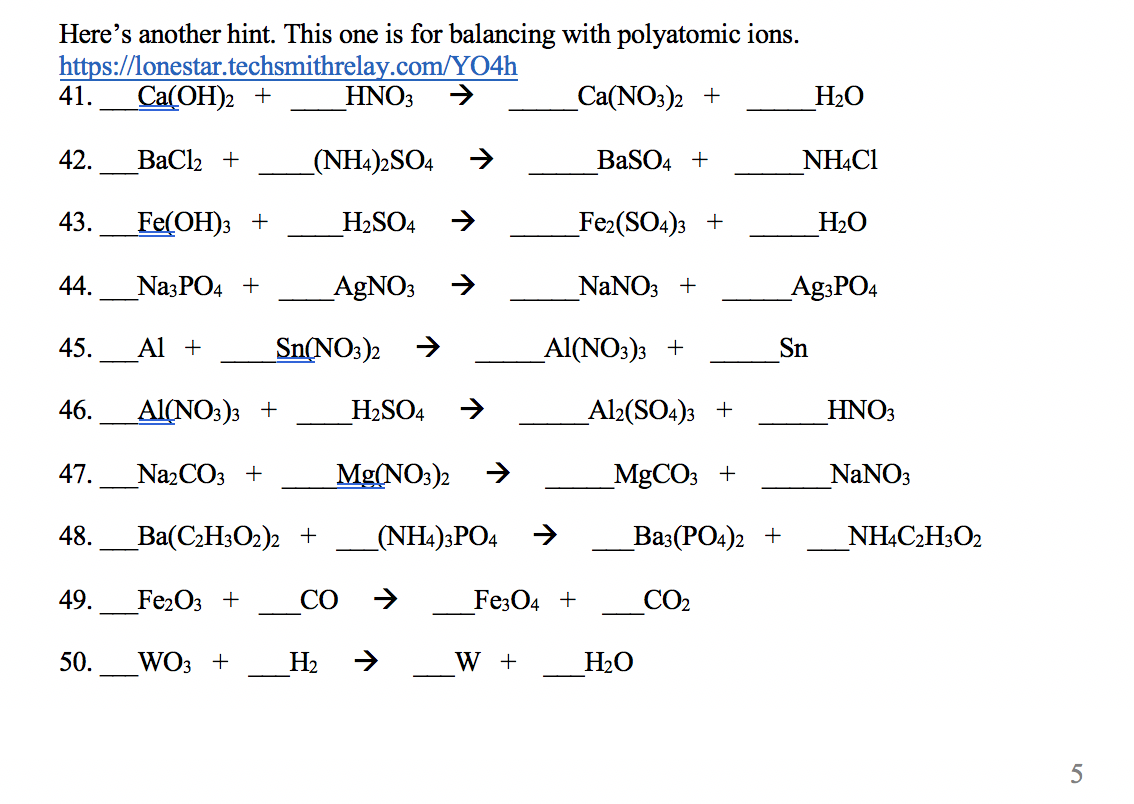
Let’s talk about the dreaded polyatomic ions. They’re like the complicated cousins of the chemical world. You know, the ones with the extra baggage and a tendency to stick together, no matter what. They show up, and suddenly, your simple equation starts looking like a math problem designed by a mischievous pixie.
And then there are the worksheets. Oh, the worksheets! They arrive, innocent enough, promising to help you master this balancing act. But sometimes, just sometimes, they feel like they’re holding out on us. They present the problems, you bravely attempt them, and then... you’re left wondering if you’ve accidentally stumbled into a parallel universe where atoms are shy and refuse to cooperate.
We’ve all been there, right? You’ve followed all the rules. You’ve counted your atoms, you’ve adjusted your coefficients, you’ve probably even whispered sweet nothings to your sulfates and nitrates. Yet, the numbers just don’t line up. It’s like trying to herd cats, but the cats are invisible and made of pure energy.
The Unpopular Opinion: Sometimes, the Answers Just… Appear.
Now, here’s where we get a little controversial. This is my personal, slightly rebellious, and utterly unpopular opinion. Sometimes, when you’re staring at a particularly stubborn polyatomic ion equation, and you’ve tried every trick in the book, the answers just… show up. Poof! Like magic. Or perhaps, like a tiny chemical fairy whispered them into your ear while you were asleep.
I’m not saying you should rely on this. Of course not. That would be academically unsound and probably get you a stern talking-to from your chemistry teacher. But admit it, haven’t you had those moments? Those blessed moments where you glance at the provided worksheet answers, and it’s like a divine revelation? You see that number 2 in front of the phosphates, and suddenly, everything clicks into place.

It’s like a jigsaw puzzle where a piece you swore was missing suddenly materializes in your hand. You might not fully understand how it got there, but you’re incredibly grateful it did. The universe, in its infinite wisdom (or perhaps a bored grader), has intervened.
Polyatomic Ions: The Gang That Stays Together
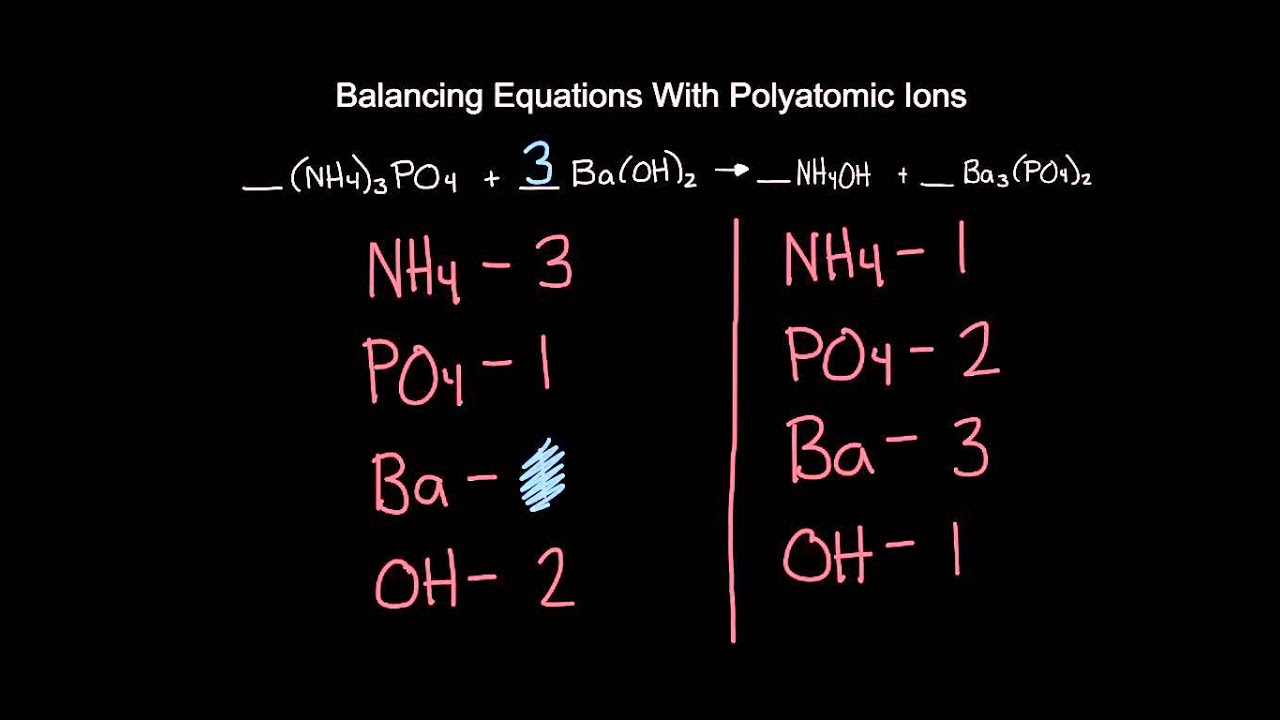
Let’s zoom in on these polyatomic ions. They’re not just random collections of atoms. They’re teams! Like a sports team, they move around in one solid unit. So, when you see a carbonate (CO₃²⁻), you don’t count the C and the O’s separately. Nope! You treat that whole CO₃ as one happy family. This is key, and frankly, a bit of a relief.
Imagine trying to balance an equation where you have to track the carbon and the oxygen individually, and then try to keep them together. It would be like trying to untangle headphone cords that have been in your pocket for a week. A tangled mess of epic proportions.
The worksheet answers, when they’re correct, respect this gang mentality. They understand that the hydroxide (OH⁻) doesn't break up easily. It’s loyal. It’s dependable. And sometimes, it’s the only thing standing between you and a completely unbalanced equation.
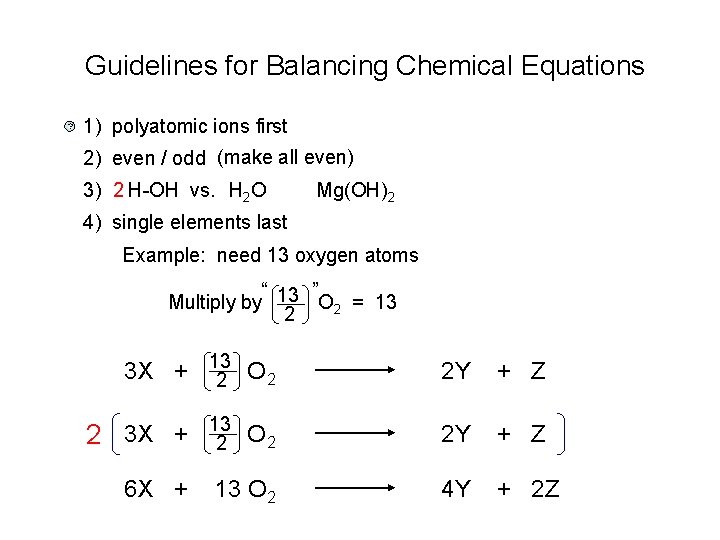
The Balancing Act: More Than Just Numbers
Balancing chemical equations, especially those with polyatomic ions, is more than just a rote exercise. It’s about understanding that matter isn’t created or destroyed. It’s just rearranged. Like playing with LEGOs, but with atoms and their charges.
And sometimes, the worksheet answers are like the instruction manual. A slightly cryptic, sometimes frustrating, but ultimately helpful instruction manual. They guide you, even if they don’t explain every single step in excruciating detail. They show you the destination, and you have to figure out the journey.
Think of it this way: when you’re learning to cook, you follow a recipe. The recipe tells you to add 2 cups of flour. You don’t question why 2 cups. You just add it. And hopefully, you end up with delicious cookies, not… well, something less delicious.
The worksheet answers for polyatomic ion equations are like those precise measurements. They are the result of someone else’s careful work, someone who has already figured out the perfect ratio. You get to benefit from their efforts, and that’s a beautiful thing.
The Moment of Truth: Checking Your Work
So, you’ve diligently worked through the problem. You’ve added your coefficients. You’ve double-checked your polyatomic ion counts. You’re ready for the moment of truth. You glance at the worksheet answers. And then… pure bliss. Everything matches. It’s a feeling of accomplishment. A small victory in the grand scheme of chemistry.

Or, it’s the opposite. You stare at the answers, then back at your work, and a cold dread washes over you. Did you miscount a phosphate? Did you forget to carry the sulfate? It’s at this point that the magic of the “pre-answered” worksheet can be both a savior and a taunter.
It’s a reminder that while we might appreciate the shortcut, true understanding comes from doing the work. But hey, sometimes a little nudge from the answer key is all you need to get it. It’s like a sudden epiphany, a light bulb moment brought to you by the power of provided solutions.
And that, my friends, is the often-unspoken joy of a well-formatted balancing chemical equations with polyatomic ions worksheet with answers. They are a testament to the fact that even in the serious world of science, a little bit of guidance (or perhaps a hint of divine intervention) is always welcome. So, the next time you’re wrestling with those tricky ions, remember this: the answers are out there. And sometimes, they’re right there on the page, waiting for you.
