Balanced Equation Of Hydrochloric Acid And Sodium Hydroxide

Okay, so you wanna talk about chemistry, huh? Don't worry, it's not all beakers and lab coats. Sometimes, it's like a tiny, exciting dance party happening right before your eyes. Today, we're diving into a classic: hydrochloric acid and sodium hydroxide doing their thing. Sounds a bit fancy, right? But trust me, it’s way cooler than it sounds.
Imagine you have hydrochloric acid. Think of it as a super-eager, a little bit aggressive, molecule. It’s a strong acid. It really wants to give away a tiny little piece of itself – a hydrogen ion, to be exact. It’s like that friend who’s always ready to share, but in a slightly… intense way.
Then you’ve got sodium hydroxide. This one is a base. It’s a bit more laid-back, but it’s also got a hidden talent. It loves to grab onto things. Specifically, it’s got a hydroxide ion that it’s just itching to pair up with something. Think of it as the patient friend, waiting for the right partner.
So, what happens when these two meet? It’s a chemical party! They can't resist each other. It’s an acid-base reaction. And this specific one is a real crowd-pleaser.
The hydrochloric acid (HCl) comes bouncing in, all excited to donate its proton (that’s the hydrogen ion, just for fun). And the sodium hydroxide (NaOH) is there, with its hydroxide ion, totally ready to receive. It’s a perfect match!
When they mix, the HCl basically says, "Here, take this hydrogen!" And the NaOH, with its hydroxide, says, "Thanks, I’ve been waiting for you!" The hydrogen from the HCl jumps over to the hydroxide from the NaOH. And poof! They form something new.
What do they form? Two things, to be exact. First, they create water. Yep, good old H₂O. The very same stuff you drink every day! How cool is that? Chemistry is literally making the stuff of life.
But that’s not all. The sodium from the sodium hydroxide and the chlorine from the hydrochloric acid are left hanging out. They’re like, "Hey, what about us?" And they decide to become buddies themselves. They form sodium chloride. Sound familiar? That’s table salt! The stuff you sprinkle on your fries. Seriously, this reaction is making everyday essentials!
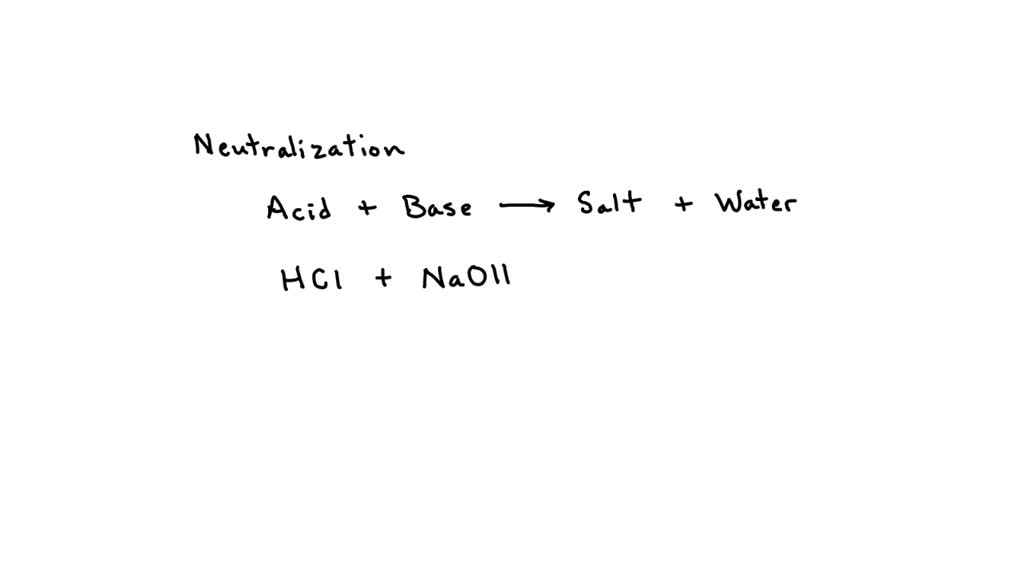
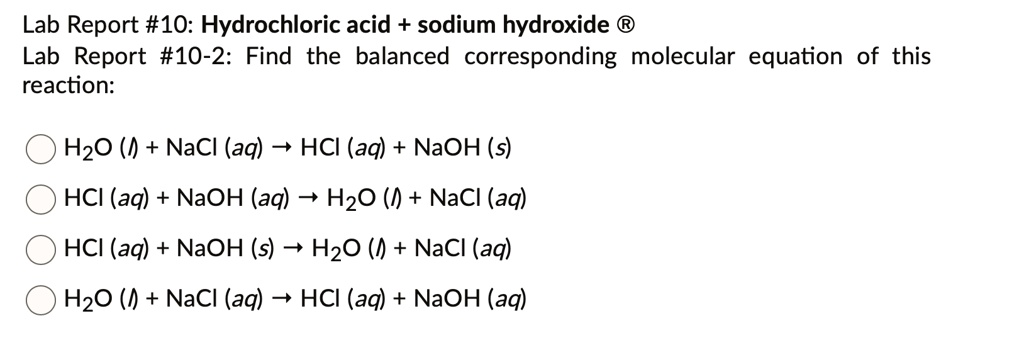
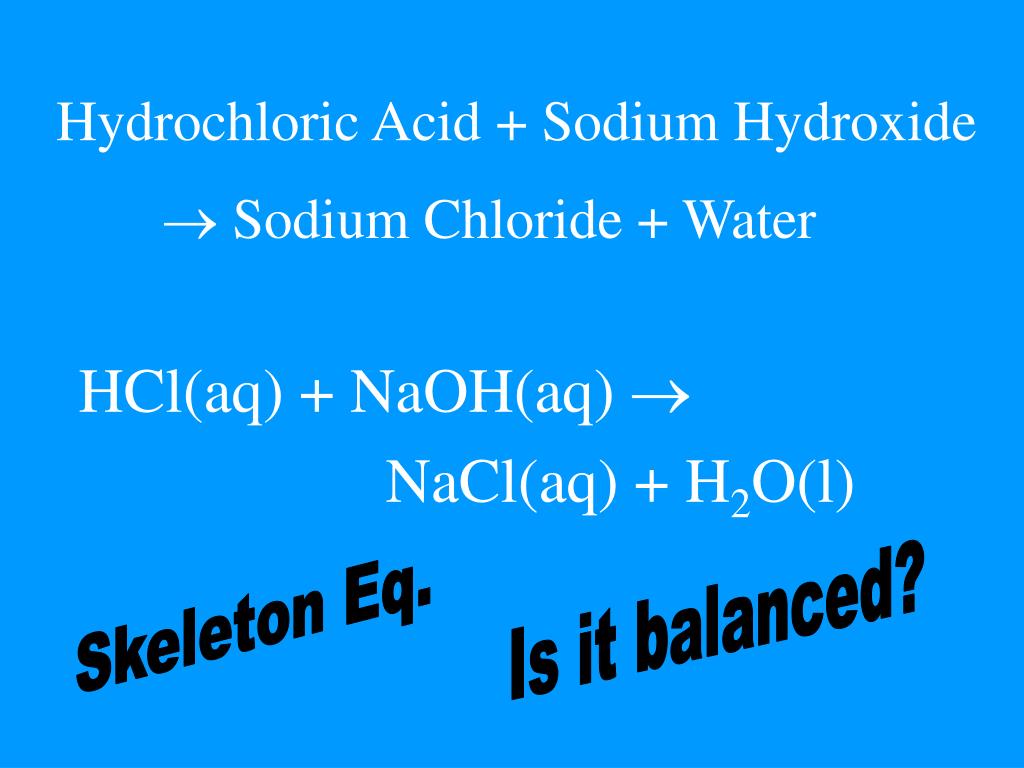
So, the whole dance looks like this: HCl + NaOH → H₂O + NaCl. Pretty neat, huh?
But here’s where it gets really fun. This reaction isn't just about making salt and water. It's about balance. You see, acids and bases have opposing personalities. When they react, they neutralize each other. They cancel each other out, in a way. That’s why it’s called a balanced equation. It’s a perfect equilibrium.

Imagine you have a super-sour lemon (that’s your acid) and a really bitter spoonful of baking soda dissolved in water (that’s your base). If you mix them, that sourness and bitterness start to disappear, right? It’s a similar idea, just with more dramatic chemical players.
The pH scale is a way we measure how acidic or basic something is. Pure water is neutral, sitting at a pH of 7. Acids have a pH below 7, and bases have a pH above 7. When HCl and NaOH react, they’re aiming for that neutral pH of 7. It’s like they’re trying to get back to a happy medium.
Now, let’s talk about the "balanced" part of the equation. It means we have the same number of each type of atom on both sides. We’ve got one sodium (Na) on the left and one on the right. We’ve got one chlorine (Cl) on the left and one on the right. And on the left, we have two hydrogens (H) and one oxygen (O) in HCl and NaOH combined. On the right, in H₂O, we have two hydrogens and one oxygen. See? Everything is accounted for. It’s like a perfect chemical accounting ledger.
This is crucial in chemistry. If an equation isn’t balanced, it means something is missing, or something extra showed up out of nowhere. And in the world of science, that just doesn't fly. It's like trying to bake a cake without measuring your flour. Disaster!
What’s also super interesting is that this reaction releases heat. It’s called an exothermic reaction. So, when these two guys get together, they get a little warm. It’s like they’re so excited to finally meet, they start to glow a little – well, figuratively speaking. In a lab, you’d definitely feel that warmth.
Think about it: you're mixing two common household chemicals, and they create something totally new and, in this case, pretty harmless, while also releasing energy. It's like a mini-magic trick. Abracadabra, salt and water!
Why is this fun to talk about? Because it’s everywhere! You might encounter hydrochloric acid in your stomach helping you digest food. Seriously, your stomach acid is basically a mild form of this stuff! And sodium hydroxide? It's in drain cleaner, which is why you have to be careful with it. It's a powerful base designed to break things down.
So, this seemingly simple equation is a gateway to understanding powerful biological processes and common household chemicals. It’s a peek into the amazing world of reactions that shape our lives.
The beauty of this reaction is its predictability. Scientists know exactly what will happen when you mix these two. They can control the amounts, predict the products, and understand the energy changes. That kind of certainty is really comforting, especially when you’re dealing with potentially reactive substances.
It’s the kind of reaction that gets taught early on because it’s a perfect example of so many fundamental chemical concepts. It’s the gateway drug of chemistry, if you will. Once you get this one, the door to understanding more complex reactions swings open.
And let's not forget the sheer elegance of it. Two molecules, seemingly so different, coming together in a perfect, balanced dance to create two essential compounds. It’s a testament to the order and structure that underlies the universe. Even at the microscopic level, there's a beautiful, predictable choreography.
So next time you see salt, or think about the water you drink, or even feel the rumble in your stomach, you can give a little nod to the humble, yet spectacular, reaction between hydrochloric acid and sodium hydroxide. It's a chemical friendship that makes the world go 'round, one balanced equation at a time. Pretty cool, right? Chemistry doesn't have to be scary; sometimes, it's just about making new friends and creating something awesome.
