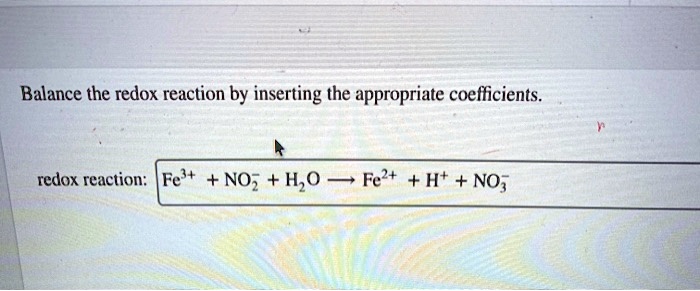
Balance The Redox Reaction By Inserting The Appropriate Coefficients

Let’s talk about something that sounds super fancy but is actually, dare I say it, a little bit… fun? We’re diving into the wild world of balancing redox reactions. Now, before you click away thinking, “Oh no, I’m not a chemist!” hang in there. This isn't about memorizing a million elements or looking like a mad scientist. This is about tidying up. Think of it like organizing your sock drawer. It feels so good when it’s done!
So, what’s a redox reaction? It’s basically a chemical party where some molecules get a little bit of what they want, and others give it up. It’s like a cosmic game of hot potato with electrons. Some atoms are handing off electrons, and others are eagerly snatching them up. It’s all about that electron transfer, baby!
Now, the real magic happens when we have to balance the equation. This is where the coefficients come in. They’re like the little numbers you sneak in front of chemical formulas to make sure everything adds up. It’s the ultimate cosmic accounting. You can't just have electrons floating around willy-nilly. Everything has to be accounted for. It’s the law of chemistry, and frankly, it’s a good one.
Imagine you’re making a really, really complicated sandwich. You have bread, peanut butter, and jelly. If you use two slices of bread, you probably want to use about the same amount of peanut butter and jelly, right? You don’t want a sandwich that’s all bread and no filling, or a glob of peanut butter on one slice and a whisper of jelly on the other. That’s just messy. Balancing is like making sure you have the perfect ratio of ingredients for the ultimate flavor explosion. In chemistry, the “flavor” is that everything is fair and square.
Sometimes, you’ll see an equation that looks like a total disaster. It’s like looking at a messy playroom after a hurricane. You’ve got a bunch of stuff scattered everywhere, and you’re thinking, “How did we even get here?” That’s when the balancing act begins. You’re the cleanup crew, the superhero of stoichiometry, swooping in to restore order.
My unpopular opinion? Balancing redox reactions is actually a little bit like solving a puzzle. You look at what’s on one side of the equation (the reactants) and what’s on the other (the products). Then, you strategically place those little coefficients, those magical numbers, to make sure the number of atoms of each element is the same on both sides. It’s a subtle dance of addition and multiplication, all in the name of chemical harmony.
Think about it: you’ve got your oxidation happening – that’s where something loses electrons. It’s like a generous friend handing over their last cookie. And then you’ve got reduction – that’s where something gains electrons. That’s the friend who happily accepts the cookie. In a redox reaction, these two things always happen together. You can’t have one without the other. It’s the ultimate chemical buddy system.
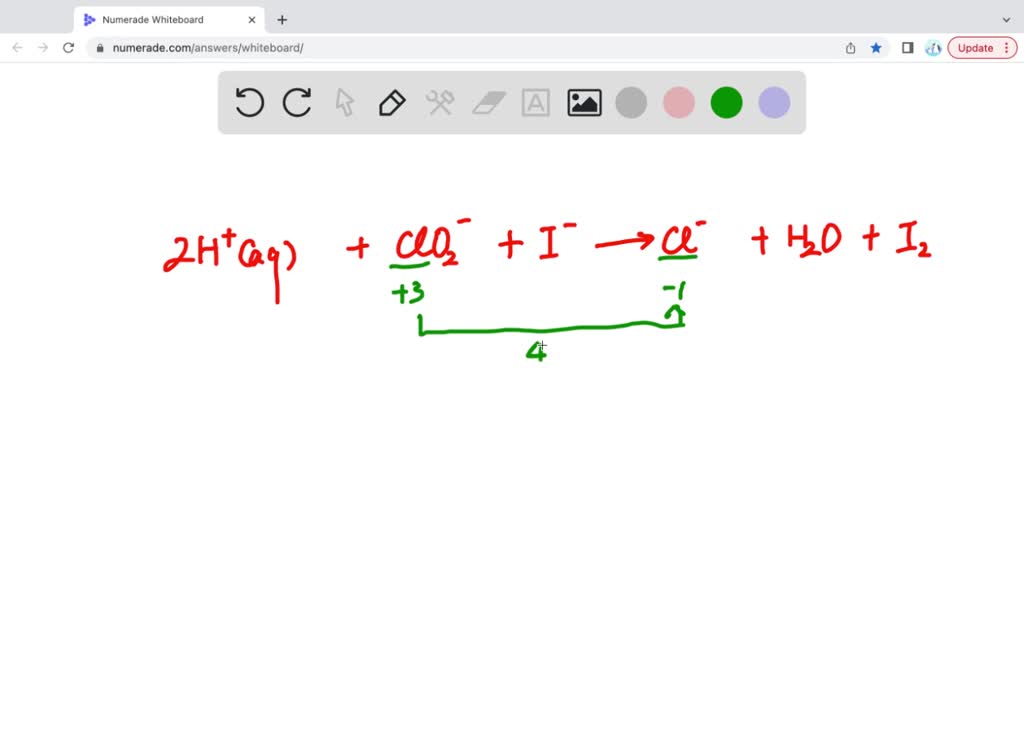
When you’re balancing, you’re essentially making sure that the electron-giving and the electron-receiving are perfectly matched. It's like a perfectly choreographed dance. If one dancer is giving too many pirouettes and the other is just standing there, the dance is off. You need an equal number of moves for everyone to make it look good. That’s what those coefficients do. They make sure every atom gets its fair share of the electron action.
Let’s say you have a particular reaction that’s just… unbalanced. It’s got more iron atoms on one side than the other, or maybe the oxygen count is all wonky. It’s like trying to count your fingers and realizing you’re off by one. It just feels wrong. That’s where the balancing coefficients become your best friends. You’re not changing the actual chemicals; you’re just changing how many of each chemical you need to make the reaction fair and square.

It's all about that perfect equilibrium, that satisfying feeling when both sides of the chemical ledger are perfectly matched.
And the best part? Once you get the hang of it, it’s surprisingly satisfying. It’s like finishing a really tough crossword puzzle or finally understanding a complicated recipe. You look at that balanced equation, and you feel a sense of accomplishment. You’ve tamed the wild chemical beasts and brought them into perfect, harmonious order.
It’s true, some people find it tedious. They see the numbers and groan. But I see a little challenge, a mental workout that’s actually kind of rewarding. It’s a small victory in the grand scheme of things, a tiny triumph over chemical chaos. So next time you’re faced with an unbalanced redox reaction, don’t despair. Grab your coefficients, put on your thinking cap, and get ready to bring some order to the universe. It’s more fun than you think!
Remember, the goal is simple: make sure all those little atoms are accounted for. It’s like a chemical headcount. No one left behind, no one getting more than their fair share. And when you get it right, when those coefficients are perfectly placed, it’s a beautiful thing. It’s chemistry at its most organized, its most balanced, and dare I say, its most elegant.
