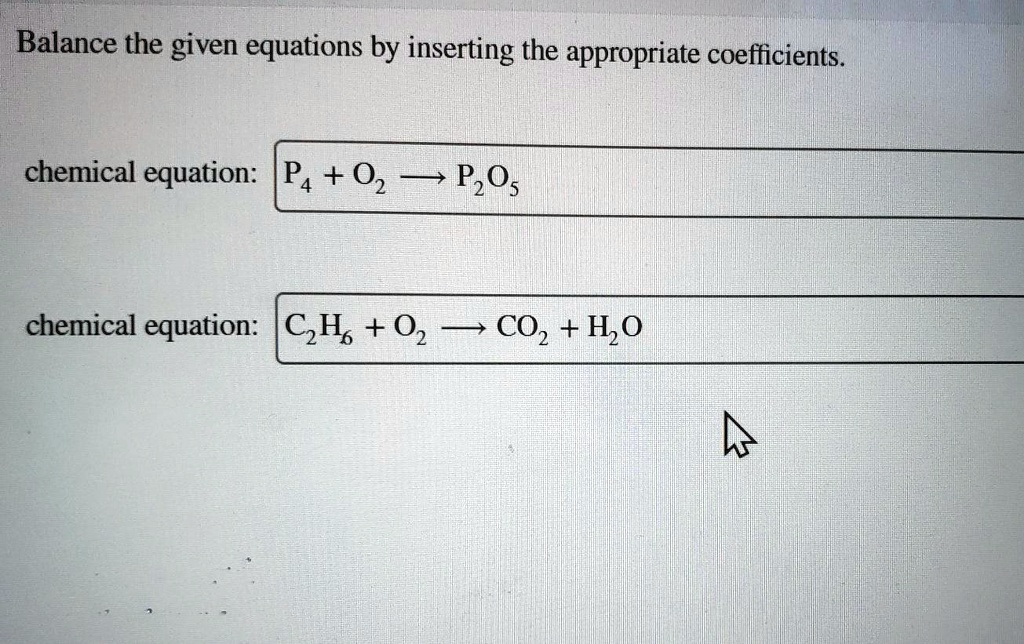
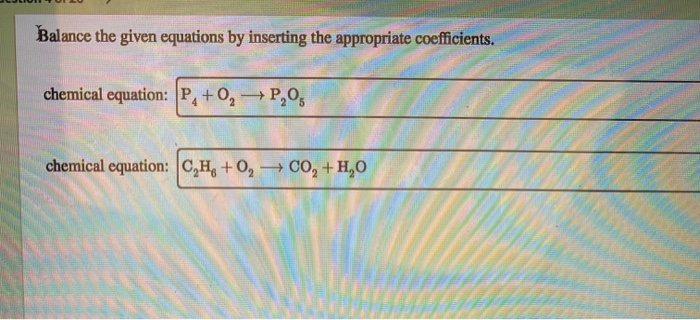
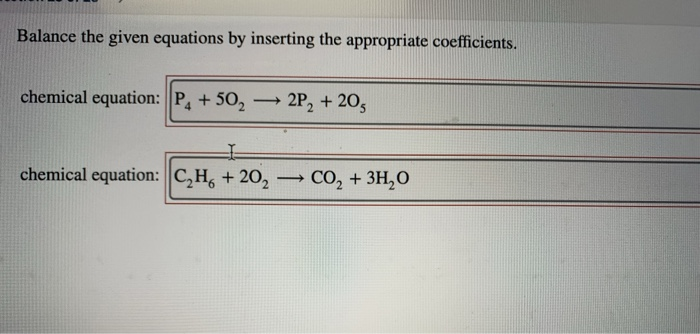
Balance The Given Equations By Inserting The Appropriate Coefficients
Hey there, you wonderful, curious human! Ever feel like life's a bit… unbalanced? Like you're juggling too many flaming torches while trying to knit a scarf and simultaneously whistle the national anthem? Well, guess what? There's a secret ingredient to a more harmonious existence, and it's hiding in plain sight, disguised as something you might have encountered in a science class: balancing chemical equations!
Now, before you picture dusty textbooks and confusing formulas, let's ditch that mental image. Think of it less like a chore and more like a fun puzzle, a little game that teaches you how to make sure everything adds up. You know how when you’re baking, you need exactly two cups of flour and one egg for that perfect cookie? It’s kind of like that, but instead of cookies, we’re talking about the tiniest building blocks of everything around us: atoms!
The Great Atom Shuffle!
So, what exactly is balancing an equation? Imagine you're playing with LEGOs. You start with a pile of red bricks and a pile of blue bricks. You then snap them together to build a cool spaceship. The basic idea behind balancing equations is that atoms are like those LEGO bricks. They don't just disappear into thin air when you mix things together, and you can't magically create more out of nowhere. What you start with on one side of the reaction has to be exactly what you end up with on the other.
Think about it! It’s like a cosmic game of conservation. The universe is a pretty tidy place, right? It likes things to be even-steven. So, when we have a chemical reaction, say, when hydrogen and oxygen decide to get cozy and form water (H₂O – yes, that stuff you drink!), the number of hydrogen atoms and oxygen atoms going into the reaction must be the same as the number of hydrogen and oxygen atoms that come out.
Why is this a big deal, you ask?
Because it’s the foundation of so much! From the air we breathe to the food we eat, from the medicines that help us to the technologies that power our lives, chemical reactions are happening constantly. And understanding how they balance helps us understand how the world works. It's like unlocking a secret code to nature's playground!
Let’s take a peek at a super simple example. Imagine we have hydrogen gas (H₂) and oxygen gas (O₂) reacting to form water (H₂O). If we just wrote it out without balancing, it might look like this:
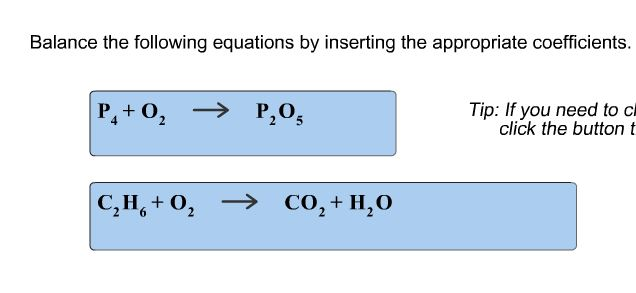
H₂ + O₂ → H₂O
Looks neat, right? But wait a minute! On the left side, we have two hydrogen atoms and two oxygen atoms. On the right side, we have two hydrogen atoms but only one oxygen atom. Uh oh! Where did the other oxygen atom go? Did it sprout wings and fly away? Nope! It’s still there, just not accounted for in our equation. That’s where the fun begins – the art of insertion!
The Coefficient Caper!
To fix this, we need to insert coefficients. These are just numbers we put in front of the chemical formulas. They act like little multipliers, telling us how many of each molecule are involved. We can't change the little numbers within the formulas (those are called subscripts, and they define what the molecule is), but we can change the big numbers in front.
So, looking at our unbalanced water equation:
H₂ + O₂ → H₂O
We have two oxygens on the left and only one on the right. To get two oxygens on the right, we need two water molecules. So, we put a ‘2’ in front of H₂O:
H₂ + O₂ → 2H₂O
Now, let’s check again. Left side: 2 hydrogens, 2 oxygens. Right side: 2 water molecules. Each water molecule has 2 hydrogens and 1 oxygen. So, 2 * (2 hydrogens) = 4 hydrogens, and 2 * (1 oxygen) = 2 oxygens. So we have 4 hydrogens and 2 oxygens on the right. We’ve got our oxygens balanced (hooray!), but now our hydrogens are out of whack (only 2 on the left, 4 on the right).
No worries! We just need to adjust the hydrogen on the left. We need 4 hydrogens to match the 4 on the right. So, we put a ‘2’ in front of H₂:
2H₂ + O₂ → 2H₂O
And voilà! Let’s count one last time: On the left: 2 * (2 hydrogens) = 4 hydrogens. And 2 oxygens. On the right: 2 * (2 hydrogens) = 4 hydrogens. And 2 * (1 oxygen) = 2 oxygens. Everything matches! We started with 4 hydrogen atoms and 2 oxygen atoms, and we ended with 4 hydrogen atoms and 2 oxygen atoms. The equation is now balanced, and the universe is a happy, tidy place once more.
It's Like a Tiny Detective Game!
See? It’s not about memorizing complex formulas; it’s about logical deduction and careful counting. It’s like being a tiny detective, meticulously ensuring that all the evidence (atoms!) is accounted for on both sides of the case. It hones your attention to detail, and honestly, there's a satisfying little ping of accomplishment when you finally get those numbers to line up perfectly.
And the best part? This skill can spill over into other areas of your life! When you approach a problem with a “let’s make sure everything balances” mindset, you might find yourself being more organized, more thoughtful about consequences, and more effective at problem-solving. It’s a subtle superpower, but a powerful one!
Think about planning a party. You need enough invitations for guests, enough food for everyone, and enough chairs for people to sit. If you don't balance your "supplies" with your "guests," things can get a little… awkward, right? Balancing equations is just the scientific version of making sure you have enough of everything for the reaction to go off without a hitch.
So, the next time you see a chemical equation, don’t shy away. Embrace it! See it as an invitation to a fascinating game, a chance to flex your brain muscles, and a way to understand the intricate dance of the universe. You'll be amazed at how much fun you can have making atoms behave!
And who knows? Once you get the hang of this, you might find yourself looking at the world with a newfound appreciation for its delicate balance and the amazing transformations that happen every single second. Keep experimenting, keep learning, and keep that sense of wonder alive. The world of chemistry is waiting to surprise you, and you've got the power to make it all add up!
