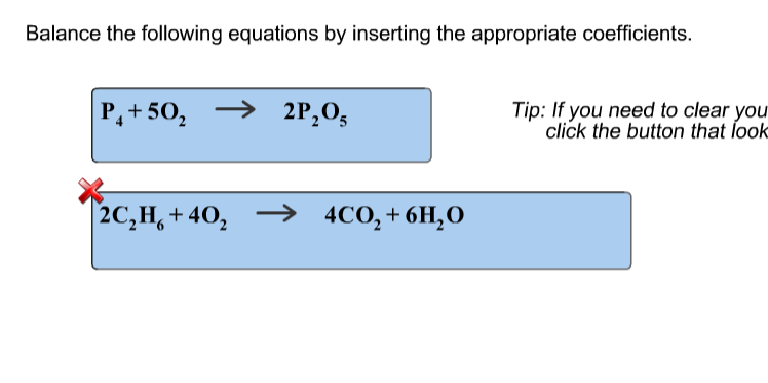
Balance The Following Equations By Inserting The Proper Coefficients
Ever found yourself staring at a jumble of letters and numbers and feeling a little bewildered? Welcome to the fascinating world of balancing chemical equations! It might sound a bit technical, but think of it like a fun puzzle, a way to make sure everything adds up perfectly. It's surprisingly relevant and, dare I say, enjoyable once you get the hang of it!
So, what's the big deal about balancing equations? At its core, it’s all about the Law of Conservation of Mass. This fundamental scientific principle tells us that in any chemical reaction, matter cannot be created or destroyed. It just rearranges itself. Balancing an equation is simply the process of ensuring that the same number of atoms of each element are present on both sides of the reaction arrow. You start with reactants (what you're mixing together) on the left, and you end up with products (what you create) on the right. Balancing makes sure the "before" and "after" have an equal count of every ingredient, atom by atom.
Why should you care? Well, understanding this concept is crucial in many fields. In chemistry education, it's a foundational skill that unlocks understanding of how molecules interact. Beyond the classroom, it's vital in industries like pharmaceuticals (to ensure accurate drug synthesis), environmental science (to track pollutants and their breakdown), and even food production (to understand how ingredients react during cooking). Imagine a chef not knowing the exact proportions of ingredients – chaos! Balancing equations is the scientific equivalent of precise recipe following.
Let's look at a simple example. When hydrogen gas (H₂) reacts with oxygen gas (O₂) to form water (H₂O), the unbalanced equation looks like this: H₂ + O₂ → H₂O. If you count the atoms, you have 2 hydrogen atoms on the left and 2 on the right, which is good. But you have 2 oxygen atoms on the left and only 1 on the right. Uh oh! To balance it, we need to add coefficients (the numbers in front of the chemical formulas). We can add a '2' in front of H₂O: H₂ + O₂ → 2H₂O. Now we have 4 hydrogen atoms on the right and only 2 on the left. So, we add a '2' in front of H₂: 2H₂ + O₂ → 2H₂O. Now, let's check: 4 hydrogen atoms on the left, 4 on the right. 2 oxygen atoms on the left, 2 on the right. Perfectly balanced!
Exploring this doesn't require a lab coat. You can find tons of online interactive tutorials that let you practice balancing equations with immediate feedback. Many educational websites offer games and quizzes that make it feel less like homework and more like a challenge. Even thinking about everyday processes, like burning wood (wood + oxygen → ash + gases + energy), can spark curiosity about the underlying chemical transformations. The key is to approach it with a sense of curiosity and a willingness to experiment with those coefficients, remembering that small adjustments can lead to big, balanced results.
