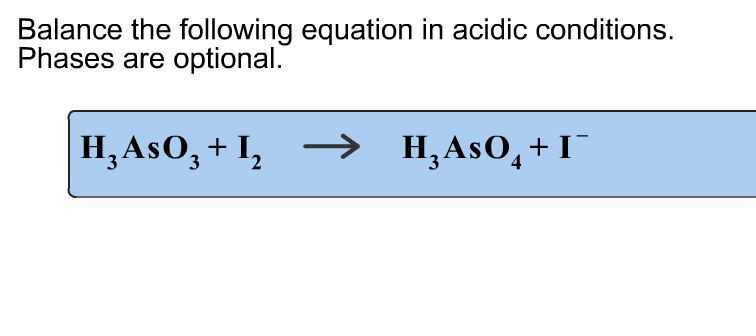
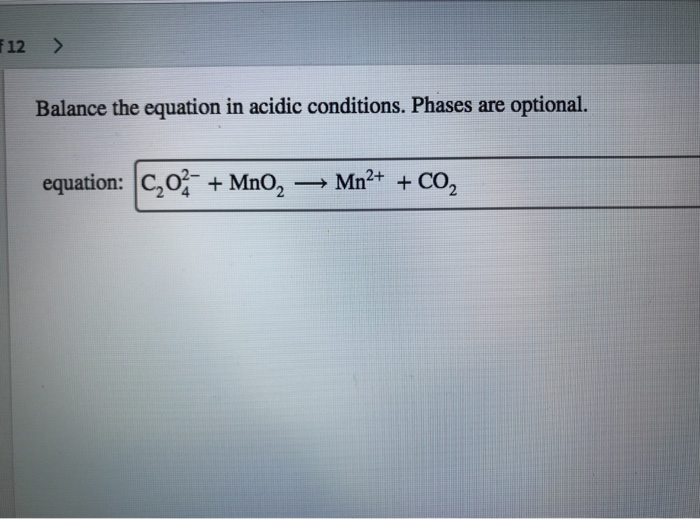
Balance The Equation In Acidic Conditions. Phases Are Optional.

Alright, gather 'round, my chemically curious comrades! Let’s chat about something that sounds super serious, like a stern librarian shushing a toddler, but is actually kinda cool. We're talking about balancing chemical equations, but with a twist. Imagine you're cooking, and suddenly, the recipe demands you make everything taste… well, sour. That’s kind of what we’re doing with our chemistry today. We’re in acidic conditions, which means we’re swimming in a pool of hydrogen ions (H⁺). Think of it as the universe’s way of saying, "Everything’s a bit tangy today!"
Now, balancing equations. We’ve all been there, right? Staring at those little formulas like they’re hieroglyphics from a civilization that only communicated in gibberish. You know, like CH₄ + O₂ → CO₂ + H₂O. It looks like a shopping list for a mad scientist who’s really bad at writing. But fear not! It’s all about making sure that what goes in the reaction is exactly what comes out. No atomic alchemy here, just good old-fashioned counting.
Usually, we just count atoms. Oxygen? Gotta have the same number on both sides. Carbon? Same. Hydrogen? You get the picture. It’s like ensuring you don’t lose any socks in the laundry. But then… bam! Acidic conditions. Suddenly, our trusty counting method needs a little… acid-itional help. (Get it? Acid-itional? I’ll be here all week. Try the veal.)
So, why the fuss about acidic conditions? Well, in acidic environments, we've got a bunch of H⁺ ions chilling out, minding their own business. These guys are like the enthusiastic but sometimes intrusive guests at your chemistry party. They can participate in the reaction, and we need to account for them. It's like trying to balance your budget when your teenager suddenly demands a new gaming console – you gotta factor that expense in!
Let’s take a peek at a typical reaction that might go down in our acidic hangout. Imagine you've got some sad, oxidized metal, and you want to cheer it up with some electrons. You might use something like this (don't worry if it looks like a dragon sneezed on a page): MnO₄⁻ + Fe²⁺ → Mn²⁺ + Fe³⁺. On the surface, it's like, "Okay, manganese and iron are doing their thing." But under the acidic hood, things get more interesting.
First off, we’re going to tackle this beast using the ion-electron method. It's a fancy name for breaking the reaction into two smaller, more manageable parts: an oxidation half-reaction (where something loses electrons, like your motivation on a Monday morning) and a reduction half-reaction (where something gains electrons, like you gaining weight after a holiday). Much less intimidating, right?
Let’s look at the iron part first, the Fe²⁺ becoming Fe³⁺. This one's a breeze. We’ve got one iron atom on each side, so that’s balanced. But the charge! We went from a +2 charge to a +3 charge. That’s a difference of +1. To balance that out, we need to add an electron (which has a -1 charge) to the right side. So, our little iron story becomes: Fe²⁺ → Fe³⁺ + e⁻. Ta-da! The atoms are balanced, and the charge is balanced. Easy peasy, lemon squeezy. Or, in our case, easy peasy, acidic squeezy.
Now for the slightly more dramatic part: the manganese. We have MnO₄⁻ turning into Mn²⁺. Let's eyeball it. One manganese on each side? Check. But the oxygens! We've got four oxygens on the left and none on the right. This is where our acidic friends, the H⁺ ions, come in to save the day. We need to balance the oxygens. And how do we do that in chemistry? With water (H₂O)! It's like using a sponge to mop up a spill, but way more controlled and less likely to involve you slipping and falling.
So, we add four water molecules to the right side to balance the four oxygens: MnO₄⁻ → Mn²⁺ + 4H₂O. Now we have atoms balanced, but our charges are looking like a messy divorce settlement. On the left, we have a -1 charge. On the right, we have a +2 charge from the Mn²⁺ and zero from the water. That’s a net +2. The difference is -3. We need to add electrons to the left side to make the charges match. But wait! We forgot about our acidic buddies.

Because we’re in acidic conditions, we can add H⁺ ions to help balance things out. We have 4 water molecules, each with 2 hydrogens, so that's 8 hydrogens on the right. To balance those hydrogens, we add 8 H⁺ ions to the left side. Our equation now looks like: 8H⁺ + MnO₄⁻ → Mn²⁺ + 4H₂O. Let’s check the charges again. On the left: (8 * +1) + (-1) = +8 - 1 = +7. On the right: (+2) + (4 * 0) = +2. Still not balanced! We need to add electrons to the side with the more positive charge (the left side) to bring it down to the other side's charge.
The difference between +7 and +2 is 5. So, we need to add 5 electrons (e⁻) to the left side. Our reduction half-reaction is now: 8H⁺ + MnO₄⁻ + 5e⁻ → Mn²⁺ + 4H₂O. Phew! Atoms balanced, charges balanced. Our acidic guests have done their duty.
Now, we have two balanced half-reactions. The iron one: Fe²⁺ → Fe³⁺ + e⁻. And the manganese one: 8H⁺ + MnO₄⁻ + 5e⁻ → Mn²⁺ + 4H₂O. Notice how one has electrons on the right (oxidation) and the other has electrons on the left (reduction)? That’s exactly what we want!
The final step is to make sure the number of electrons lost in the oxidation equals the number of electrons gained in the reduction. We lost 1 electron in the iron reaction and gained 5 electrons in the manganese reaction. To make them match, we need to multiply the entire iron reaction by 5. So, Fe²⁺ → Fe³⁺ + e⁻ becomes 5Fe²⁺ → 5Fe³⁺ + 5e⁻.

Now we have 5 electrons on the right of the iron equation and 5 electrons on the left of the manganese equation. They’re ready to cancel each other out like rival superheroes finally realizing they’re on the same team. We add the two balanced half-reactions together, canceling out the electrons:
5Fe²⁺ → 5Fe³⁺ + 5e⁻
8H⁺ + MnO₄⁻ + 5e⁻ → Mn²⁺ + 4H₂O
----------------------------------------------
8H⁺ + MnO₄⁻ + 5Fe²⁺ → Mn²⁺ + 5Fe³⁺ + 4H₂O
And there you have it! A beautifully balanced chemical equation, complete with our acidic H⁺ ions playing a crucial role. It’s like a perfectly orchestrated dance where every atom, every ion, and every electron knows its steps. And all because we weren't afraid to get a little… acidic.
So, next time you’re staring at a chemical equation and the conditions are described as "acidic," don’t panic. Just remember the H⁺ ions are your friends, water is your mop, and balancing is just a fancy way of making sure nobody’s cheating. It’s not just about making sure the atoms are there; it’s about making sure the charge is there too, especially when you’re in a soupy, sour situation. Now, who wants to try balancing in basic conditions? That’s a whole other kettle of… electrons.
