Balance Each Redox Reaction Occurring In Basic Aqueous Solution

Hey there! Ever stare at a chemical equation and think, "What in the heck is going on here?" Especially when it's in a basic solution? Yeah, I feel you. Today, we're diving into the wild world of redox reactions in basic aqueous solutions. Sounds fancy, right? But trust me, it's way more fun than it sounds. Think of it like a chemistry dance-off, but with electrons!
So, what's the big deal with redox? It's all about oxidation and reduction. Oxidation is like giving away electrons. Reduction is like snatching them up. It's a give-and-take, a never-ending electron tango. And when this happens in a basic solution? Oh boy, things get a little spicy.
Basic solutions are, well, basic. Think of stuff like baking soda or drain cleaner. They've got a surplus of hydroxide ions (OH⁻). These little guys are like the party organizers of the reaction. They're not just sitting there; they're actively involved in the electron drama. It’s like they brought the disco ball and the funky music to the electron party!
Balancing redox reactions is like solving a puzzle. But this puzzle involves electrons, atoms, and making sure everything adds up. It’s a bit like making sure you have enough pizza slices for everyone at a party. Everyone needs their fair share of electrons, and we gotta make sure nobody’s left out.
In acidic solutions, it's kinda straightforward. You balance atoms, then balance charge with H⁺, and finally, you get water. Easy peasy. But in basic solutions? Nah, that’s too simple for us, right? We gotta add a little extra flair.
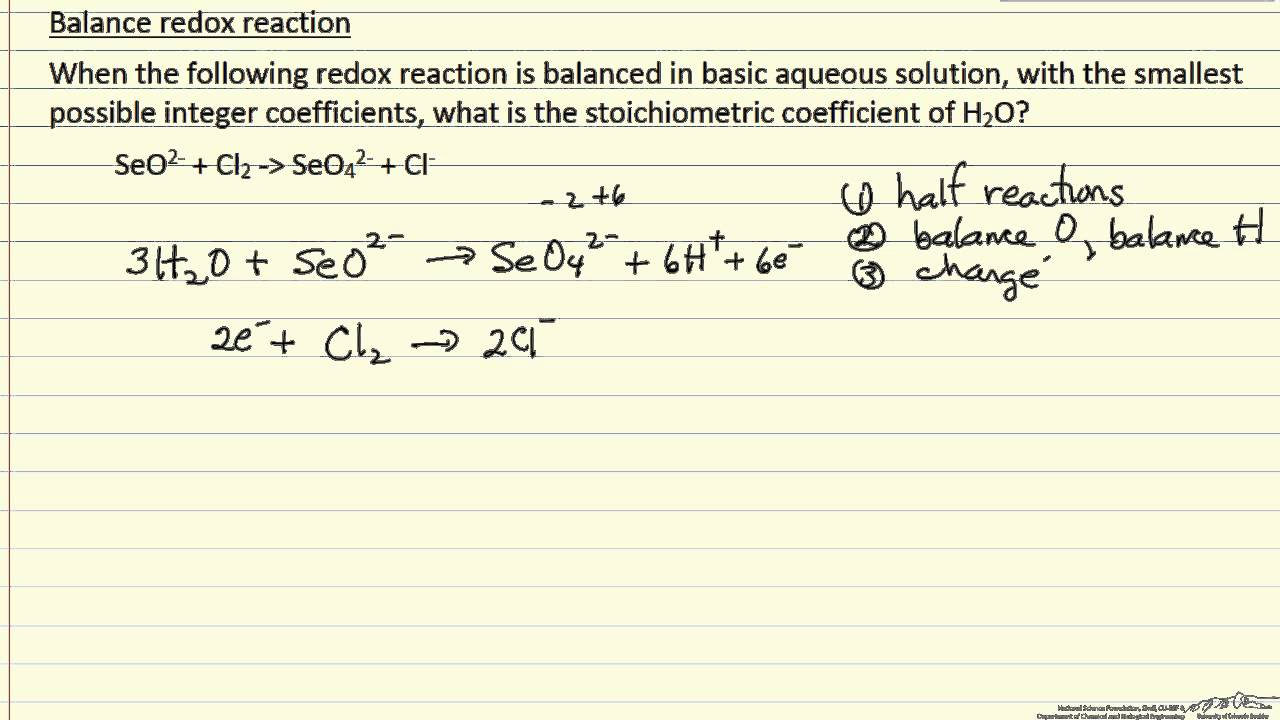
The trick in basic solutions is that we can't just add H⁺ willy-nilly. Remember, it's basic. Adding H⁺ would be like inviting a cat to a dog party. It just doesn't fit! Instead, we use a super cool trick.
The Magical OH⁻ Trick!
Here's where the fun really starts. We first pretend we're in an acidic solution. Yeah, you heard me. We balance the reaction as if it were acidic. We balance atoms, then use H⁺ and H₂O. It’s like doing the steps for a dance and then realizing you’re at the wrong venue. But don’t panic! That's where the magic happens.
Once we have our "acidic" balanced equation, we realize our mistake. Oops! We've got all these H⁺ ions hanging around. They’re like the awkward guests who showed up to the wrong party. So, what do we do? We add hydroxide ions (OH⁻) to both sides of the equation. And here's the kicker: we add enough OH⁻ to cancel out all the H⁺ ions.
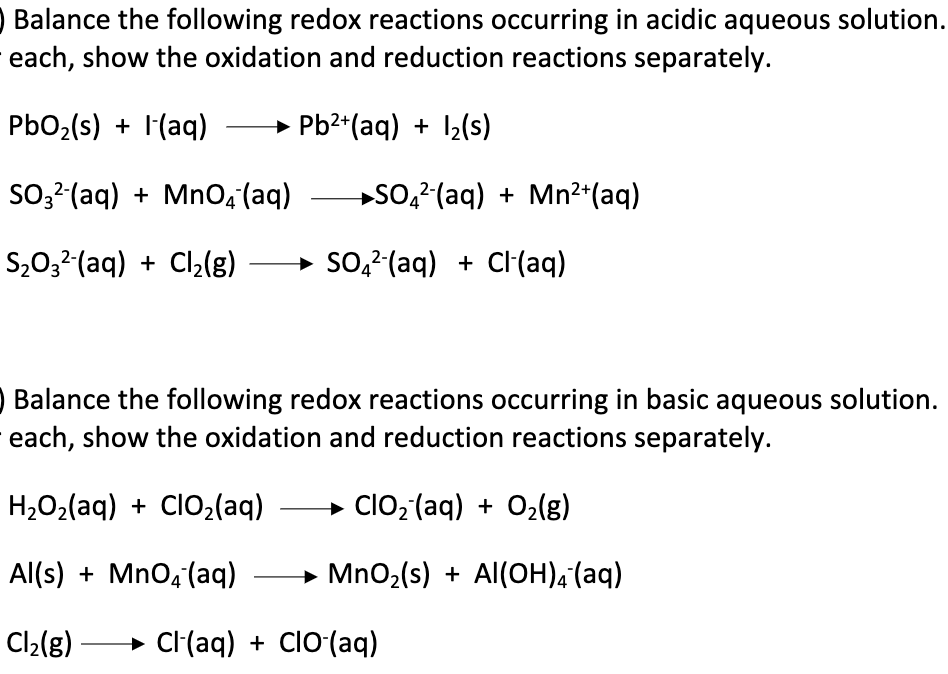
Think of it this way: H⁺ and OH⁻ are like frenemies. When they meet, they immediately decide to form water (H₂O). Poof! They disappear from the equation and become a more stable, less intrusive molecule. It's like a chemistry magic trick, turning awkward H⁺ guests into a nice, neutral glass of water.
So, we add OH⁻ to both sides. On one side, the OH⁻ teams up with the H⁺ to form water. On the other side, the added OH⁻ just… hangs out. It’s like bringing a date to a party where everyone else is single. They just join the crowd.
Then, we do a final sweep. We simplify the equation by canceling out any excess water molecules that appear on both sides. It's like tidying up after the party, making sure everything is neat and tidy. And voilà! You have your perfectly balanced redox reaction in a basic solution.

Why is this so cool?
Because it’s a system! Chemistry isn't just random chaos. There are rules, and there are clever workarounds. This method of balancing in basic solutions is a testament to that. It shows how chemists can adapt and find solutions (pun intended!) to tricky problems.
Plus, let’s be honest, manipulating electrons sounds like a superpower. And when you can balance these reactions, you’re basically a super-powered electron juggler. You’re making sure every electron is accounted for, keeping the chemical universe in balance.
Think about where these reactions happen. They're crucial in batteries, in how our bodies digest food, and even in water treatment. So, while you're busy balancing equations, you're actually touching on processes that are fundamental to life and technology. Pretty neat, huh?
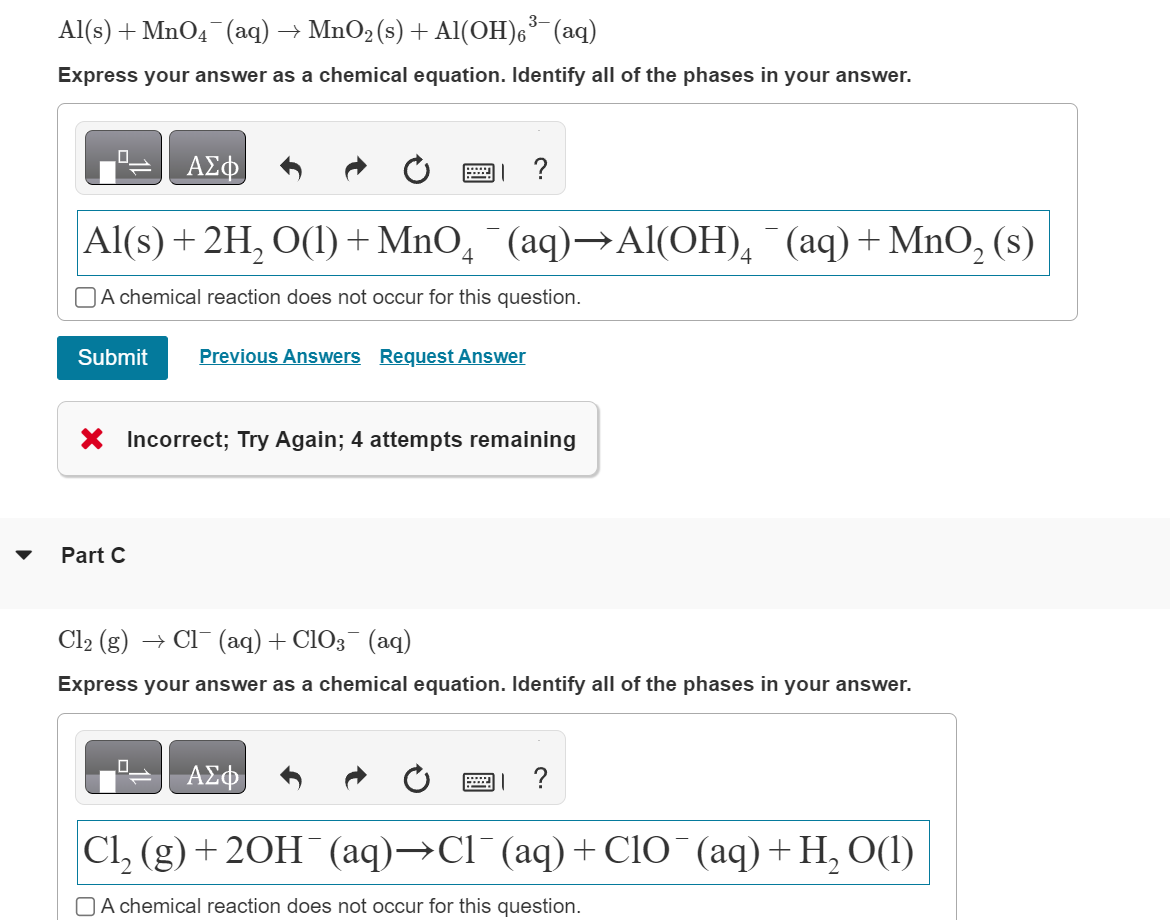
And the quirky facts? Well, sometimes you'll see some really weird intermediates pop up. It's like, "Where did that molecule come from?" But with a bit of patience and the OH⁻ magic trick, they all fall into place.
It’s also a fantastic exercise for your brain. It’s like doing a Sudoku, but with elements and charges. It trains you to think logically, to follow steps, and to appreciate the underlying order in what can seem like complex reactions.
So, next time you see a redox reaction in a basic solution, don't groan. Smile! Grab your virtual beaker and pipette, and get ready for the electron dance. Remember the OH⁻ trick, and you’ll be balancing like a pro in no time. It’s a little bit of magic, a lot of logic, and a whole lot of fun. Happy balancing!
