Atoms Whose Outer Electron Shells Contain Eight Electrons Tend To
Hey there, ever wondered why some things in life just stick together, while others seem to drift apart? Think about your favorite comfy armchair – it’s been in the same spot for ages, right? Or maybe you’ve got a friend you’re just super close to, always on the same wavelength. Turns out, there’s a tiny, invisible reason for a lot of this stability, and it all comes down to what’s happening at the absolute tiniest level of matter: atoms.
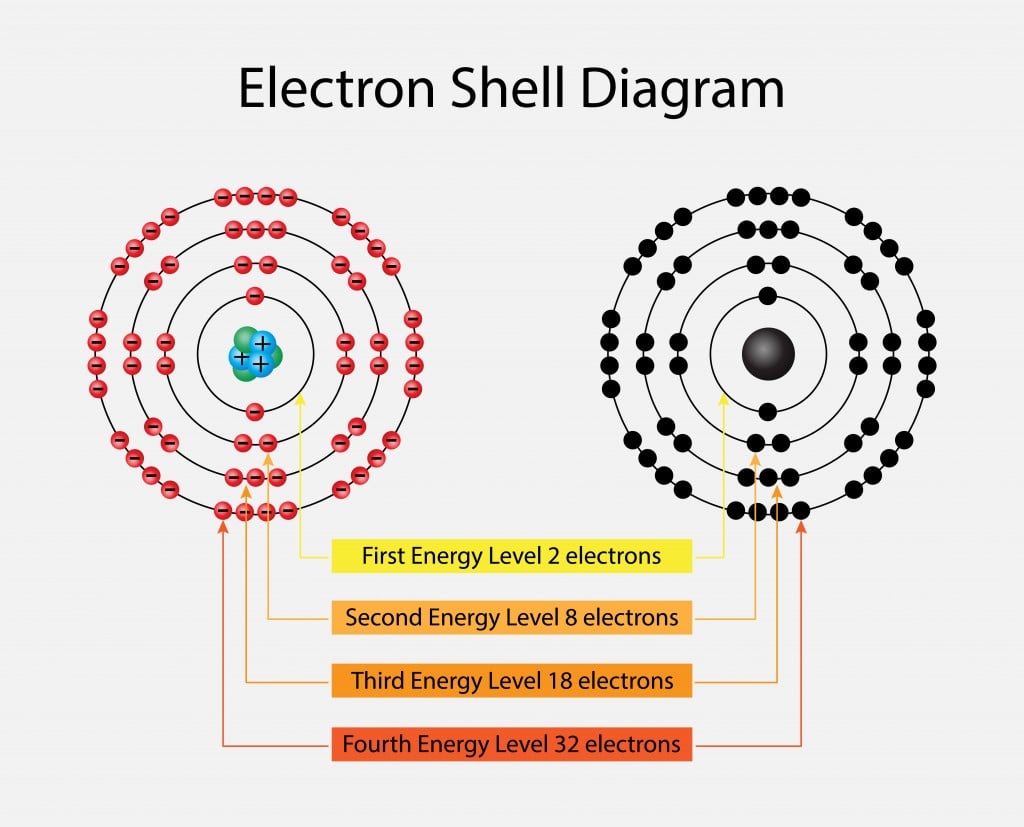
Now, before you start picturing complicated science diagrams, let’s keep it super chill. Imagine atoms as little solar systems. In the center, you’ve got the nucleus (like the sun), and zipping around it are electrons (like planets). These electrons don’t just hang out anywhere; they orbit in specific “shells,” kind of like different lanes on a racetrack.
What’s really cool is that these shells have a limit to how many electrons they can hold. The innermost shell, the one closest to the nucleus, is happy with just two electrons. Think of it as a cozy two-seater car. But the shells further out? They can fit more. And here’s the magic number: eight.
When an atom’s outermost electron shell has exactly eight electrons, it’s like it’s found its perfect match, its happy place. It’s achieved what scientists call a stable configuration. Imagine a perfectly balanced seesaw with just the right weight on each side. It’s not wiggling, it’s not trying to move; it’s just… content. These atoms are like those super chill friends who are perfectly happy just being themselves, not needing any extra drama or company to feel complete.
So, what does this mean for us everyday folks? Well, a ton! These stable atoms are the building blocks of a lot of the world around us. Because they’re so content, they’re not usually looking to grab or give away electrons like a frantic shopper trying to complete a set. They’re more likely to just… be.
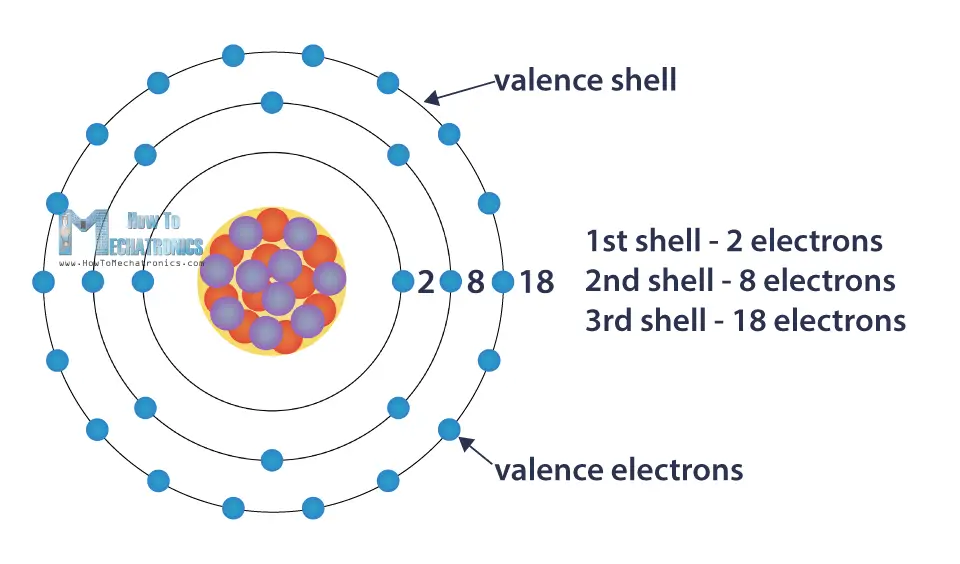
Think about noble gases. You might have heard of them – helium, neon, argon. These are the superstars of electron shell satisfaction. Their outer shells are already rocking that magical number of eight (except for helium, which is happy with two in its first shell, which is its only shell). That’s why neon signs glow with such steady, unwavering light. The neon atoms are just chilling, doing their stable thing. They don’t need to react with anything to feel better; they’re already in their ultimate comfort zone.
It's like having a perfectly brewed cup of tea. It’s just right. You don’t need to add more sugar or milk; it’s already perfect. These atoms are that perfect cup of tea. They don’t need to bond with other atoms to try and achieve some kind of electron-based enlightenment. They’ve already arrived.
This stability is why some elements are so unreactive. They’re like the introverts of the atomic world. They’re perfectly happy in their own little atomic bubble. They don’t go around looking for trouble or trying to make friends by forming chemical bonds. They're the folks who are perfectly fine at home with a good book and a cup of cocoa, not feeling the need to go to a big, bustling party.
+with+8+electrons+(Octet+rule)..jpg)
On the flip side, atoms that don’t have eight electrons in their outer shell are a different story. They’re like that friend who’s always looking for something new, a bit of excitement. They’re either trying to gain an electron to fill that shell up, or they’re trying to give away an electron to get to a previous, stable shell. This is where all the action happens in chemistry!
Imagine you have one cookie left, and your friend has none. You might be tempted to share, or your friend might be really tempted to ask for it. That’s a bit like how atoms with incomplete outer shells behave. They’re looking to achieve that magical number of eight.

But the atoms with eight electrons? They’re like the person who just finished their entire cookie. They’re satisfied. They’re not looking for more, and they don’t have any extra to give away. They’re just… complete. This makes them incredibly useful in situations where you want something to be reliable and unchanging.
Think about the materials we use every day that need to last. Metal in your car? Sturdy buildings? Your trusty smartphone screen? Many of the elements that make up these things are on their way to or already possess this desirable eight-electron shell. They form strong, stable bonds with each other, creating materials that can withstand the tests of time and use.
It’s like building with really good LEGO bricks. When the bricks fit together perfectly and are made of strong plastic, your creation stays put. The atoms with eight electrons in their outer shell contribute to that "strong plastic" of the molecular world, making things durable and dependable.

So, why should you care about this? Because understanding this simple principle helps us appreciate the world around us on a much deeper level. It explains why some gases are safe to breathe (like the nitrogen and oxygen in the air, which have stable electron configurations), and why others are dangerous. It’s why we have incredible technologies, from medicine to electronics, that rely on the predictable behavior of atoms and molecules.
It's the quiet, behind-the-scenes reason why your water doesn’t spontaneously decide to become something else, or why the air you’re breathing stays as air. It’s the unseen stability that allows for the consistent, reliable world we often take for granted.
The next time you see a beautiful neon sign, or admire the strength of a steel beam, or even just enjoy the fresh air, give a little nod to those atoms with their perfectly balanced outer shells. They’re the unsung heroes of stability, quietly making our world a predictable and wonderful place to live. It’s a little bit of science magic, happening everywhere, all the time, making sure things just… work.
