At What Temperatures Will The Following Processes Be Spontaneous

Get ready to dive into the wonderfully wacky world of spontaneity! You know, those things that just happen without you having to force them? Like that irresistible urge for a second slice of pizza, or the way your cat spontaneously decides your keyboard is the warmest spot in the house. Well, it turns out there are some super cool scientific reasons behind why things just decide to go for it, and it all boils down to temperature!
We're going to explore some everyday processes and discover at what temperatures they're basically saying, "Let's do this!" It's like a temperature-powered party starter for the universe, and we're all invited!
The Coffee Cup Conundrum: When Does Your Brew Get Chilly?
Imagine your perfectly brewed coffee. Bliss, right? But then, time happens. And eventually, that delightful warmth fades into a lukewarm meh. This is a classic case of spontaneous heat transfer, and it's happening all around us, all the time.
Think of it like a tiny energetic party where the hot coffee molecules are super hyped up and bouncing all over the place. The cooler air around it is a bit more chill. These energetic coffee molecules can't help but share their enthusiasm, bumping into the air molecules and passing on their heat. It's like a social butterfly spreading good vibes (and heat)!
This particular spontaneous dance happens at pretty much any temperature above absolute zero. That's the super-duper cold theoretical point where everything stops moving. So, your coffee will always spontaneously cool down to match its surroundings. It’s a fundamental rule of the universe: heat loves to spread out!
So, the moment you pour that hot coffee, the spontaneous cooling begins. It's not waiting for a specific temperature threshold; it's on a perpetual mission to reach equilibrium. Even a slightly warm drink will lose heat to a cooler room. It’s nature’s way of saying, "Let's all be the same temperature, shall we?"
The Melting Marvel: Ice Cream's Sunny Disposition
Ah, ice cream. The ultimate treat for a hot day. But have you ever left it out for just a minute too long? Suddenly, that firm, scoopable delight transforms into a sad, soupy puddle. That, my friends, is spontaneous melting in action!

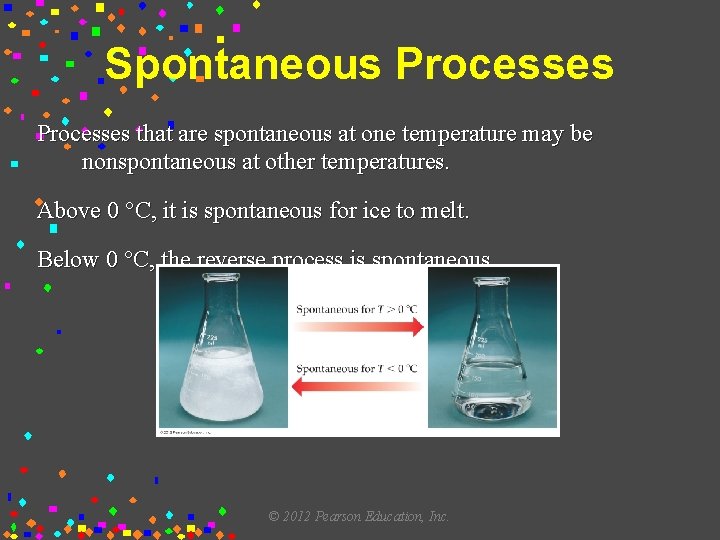
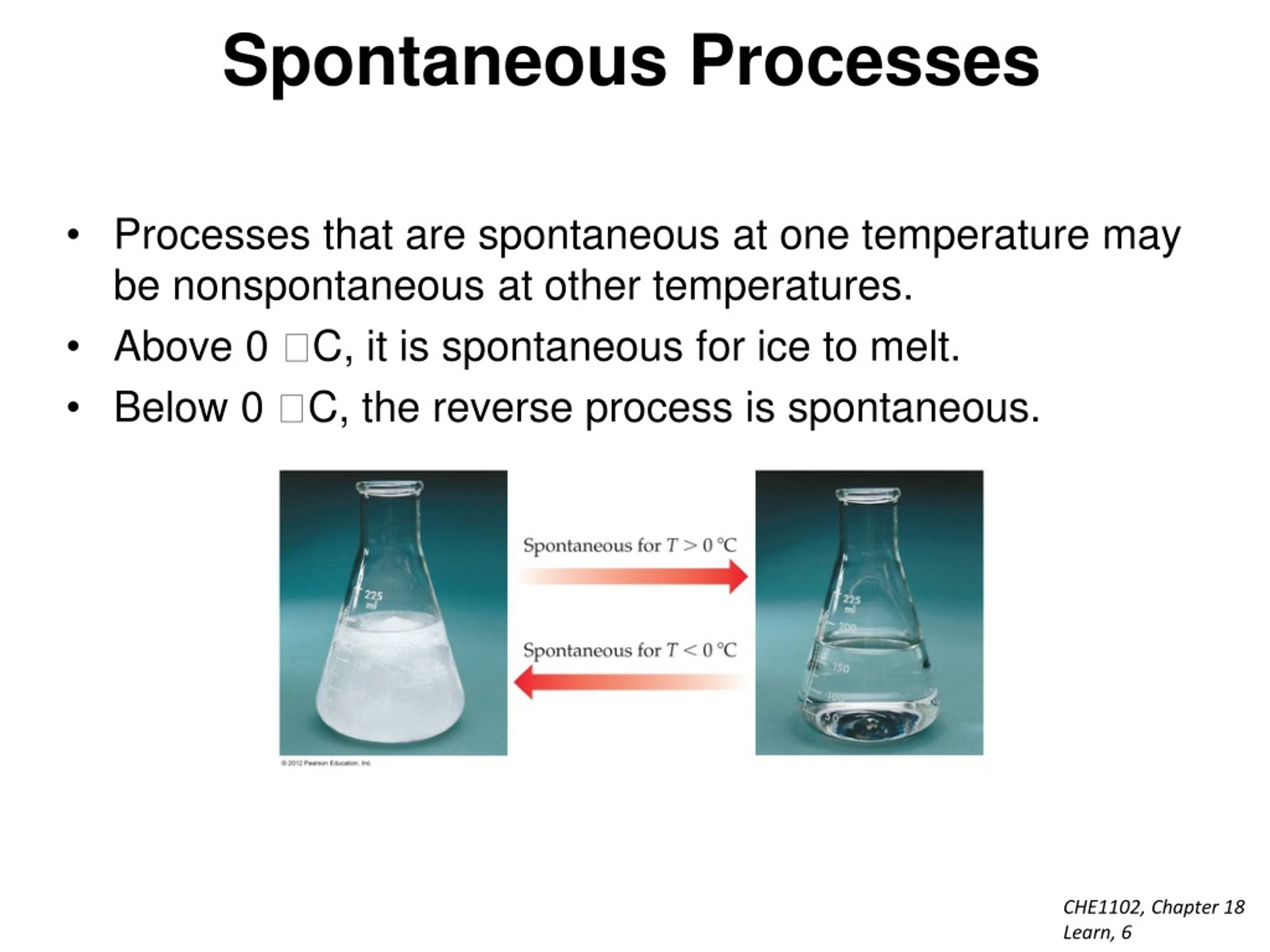
Ice melting is like a bunch of frozen water molecules saying, "You know what? This is a bit too restrictive. Let's loosen up and flow a little!" They need a bit more energy to break free from their rigid ice structure and start moving around more freely, like dancers on a crowded dance floor.
This happy dance of melting typically starts to get going at temperatures above the freezing point of water, which is 0 degrees Celsius (32 degrees Fahrenheit). So, as soon as your ice cream is out of the freezer and in a place warmer than that, the spontaneous transformation is underway. It’s like a backstage pass to freedom for those water molecules!
The warmer it gets, the faster this spontaneous process occurs. A scorching summer day is like throwing a massive party for melting, while a cool, crisp autumn day might let your ice cream linger a bit longer before its inevitable gooey fate. It’s a temperature-controlled countdown to deliciousness (or disaster, depending on your perspective!).
The Dissolving Delight: Sugar in Your Tea
Who doesn't love a little sweetness in their cuppa? When you toss a sugar cube into your hot tea, it doesn't just sit there looking pretty. Nope, it dissolves, disappearing into the liquid like a magic trick. This is the magic of spontaneous dissolution!
Imagine sugar molecules as little partygoers who are super excited to mingle with the tea molecules. They love to spread out and make new friends. The hotter the tea, the more energetic these interactions become, making it easier for the sugar to break apart and mix in.
Dissolving can happen at many temperatures, but it becomes significantly more spontaneous and faster as the temperature increases. While sugar will dissolve in cold water, it’s a much slower, more laid-back affair. Think of it as a leisurely stroll versus a full-on sprint.
For most common substances like sugar, this process is generally spontaneous at room temperature and above. The warmer your drink, the quicker and more complete the dissolution. It’s like the sugar molecules are saying, "Let's get this party started, and the warmer it is, the better!"
The Rusting Racket: When Your Bike Gets a Bit Brown
Now, not all spontaneous processes are as delightful as melting ice cream. Have you ever noticed how your shiny metal bike can develop a reddish-brown layer if left out in the rain? That's spontaneous rusting, and it's a chemical reaction that's just itching to happen!
Rusting is essentially iron in metal getting super excited about oxygen and water. It's like a chemical love triangle where iron, oxygen, and water all want to combine. This combination forms iron oxide, which we know and love (or perhaps don't love) as rust.
This particular spontaneous reaction tends to occur at temperatures above freezing. The presence of moisture is a huge catalyst, making it happen much more readily. Think of it as the necessary ingredients for a chemical explosion of brown!
While it can happen slowly at cooler, damp temperatures, it speeds up considerably as things warm up. So, a hot, humid day can be a rust's best friend, accelerating the process. It’s a rather unglamorous but undeniably spontaneous transformation, happening without any human intervention whatsoever.
The Baking Bonanza: Turning Dough into Deliciousness
From fluffy bread to perfectly browned cookies, baking is a magical process powered by heat. When you pop that dough into the oven, you're setting the stage for a whole symphony of spontaneous chemical changes.

These changes involve complex reactions where ingredients transform. Think of proteins and starches in the dough getting all excited and rearranging themselves. They brown, they puff up, they develop those amazing flavors and textures we crave.
The temperature required for these spontaneous baking transformations is typically around 150-200 degrees Celsius (300-400 degrees Fahrenheit). This is the sweet spot where the molecules are energized enough to undergo significant, delicious changes.
It’s a precise dance of heat and chemistry, where the oven’s warmth provides the perfect environment for these spontaneous reactions to create edible art. Without that specific temperature range, your dough would remain just that – dough. It's a temperature-dependent miracle!
The Verdict: Temperature is the Ultimate Mood Setter!
So there you have it! From the humble coffee cup to the magical oven, temperature plays a starring role in whether things just decide to go for it. It's the universe's way of setting the mood for change, from delightful melting to slightly less delightful rusting.
The next time you see something change or transform without you lifting a finger, remember the power of temperature! It’s a constant, unseen force making the world around us a dynamic and ever-changing place. And that, in itself, is pretty darn spontaneous and exciting!
