Arrange This Isoelectronic Series In Order Of Decreasing Radius

Hey there, fellow science enthusiasts! Ever looked at a bunch of atoms and thought, "Man, they all look kinda the same, but are they really?" Well, buckle up, buttercup, because today we're diving into a super cool concept called an isoelectronic series. Don't let the fancy name scare you; it's actually pretty straightforward and, dare I say, fun!
So, what's the deal with isoelectronic series? Think of it like this: imagine a bunch of people who all have the exact same number of fingers and toes. They might look different on the outside – some tall, some short, some with funky hairstyles – but their fundamental "building blocks" (in this case, digits) are identical. In chemistry, isoelectronic species are atoms or ions that have the same number of electrons. Yep, that's it! Same electron count, even if they're different elements or have different charges.
Why is this important, you ask? Because when things have the same number of electrons, we can start comparing them based on other factors, like how much oomph the nucleus has to pull on those electrons. And that, my friends, leads us to our main mission for today: arranging these isoelectronic critters in order of decreasing radius. Radius, in this context, is just a fancy way of saying "how big" the atom or ion is. Think of it as their atomic waistline!
Now, you might be wondering, "How do I even find an isoelectronic series?" Good question! The trick is to look for elements that have lost or gained electrons to achieve a stable electron configuration, often resembling that of a noble gas. Noble gases are like the cool kids of the periodic table, they're all chill and don't really like to mess with anyone because their electron shells are full. So, other atoms try to be like them!
Let's take a common example, the isoelectronic series with 10 electrons. This usually involves the second-period elements. We're talking about ions like O2-, F-, Ne, Na+, Mg2+, and Al3+. See? All of them have a grand total of 10 electrons. Pretty neat, right?
Here’s a little secret: when you have the same number of electrons, the biggest factor influencing their size (radius) is the number of protons in the nucleus. More protons mean a stronger positive charge, and that positive charge is like a super-powered magnet pulling on those negatively charged electrons. The stronger the pull, the tighter the electrons are held, and the smaller the atom or ion becomes.
So, to arrange them in order of decreasing radius (from biggest to smallest), we need to go from the one with the fewest protons to the one with the most protons. It's like a tug-of-war for electrons, and the team with the most players on their side (protons) wins, pulling the electron cloud in closer!
Let's break down our 10-electron series:
Oxygen (O2-)
Oxygen normally has 8 protons. When it gains two electrons to become O2-, it still has 8 protons. But now it has 10 electrons. Those 8 protons are trying to keep 10 electrons in check. That's a lot of electrons for a relatively small nuclear charge. So, O2- has more "elbow room" for its electrons, making it the largest in this series. It's like having a big family in a small apartment – things get a bit spread out!

Fluorine (F-)
Fluorine has 9 protons. When it gains one electron to become F-, it also has 10 electrons. Now, we have 9 protons trying to hold onto those 10 electrons. That's a bit more of a pull than oxygen has. The protons are starting to get a little more serious about keeping those electrons close. So, F- is going to be smaller than O2-.
Neon (Ne)
Neon is a noble gas and has 10 protons. In its neutral state, it has 10 electrons. Here, the number of protons (10) exactly matches the number of electrons (10). This is a stable configuration, and the pull is pretty balanced. Neon is going to be smaller than the negatively charged ions because there's no "extra" electron pushing outwards, and the positive charge is directly balanced by the negative charge.
Sodium (Na+)
Sodium usually has 11 protons. When it loses one electron to become Na+, it now has 10 electrons. We have 11 protons now trying to hold onto just 10 electrons. That's a stronger pull! The nucleus is really tightening its grip. So, Na+ is going to be smaller than Neon. It's like the principal of the school keeping a closer eye on a smaller group of students.
Magnesium (Mg2+)
Magnesium has 12 protons. When it loses two electrons to become Mg2+, it still has 10 electrons. Now, we have 12 protons pulling on those 10 electrons. That's a significantly stronger nuclear charge! The electrons are going to be held very tightly. Mg2+ is going to be smaller than Na+. The proton power is really kicking in!
Aluminum (Al3+)
And finally, we have Aluminum. Aluminum has 13 protons. When it loses three electrons to become Al3+, it ends up with 10 electrons. Now, we have a whopping 13 protons trying to manage just 10 electrons. That's the most protons we have in our 10-electron series. The nuclear attraction is at its strongest here, and the electrons are pulled in as tightly as possible. Therefore, Al3+ is the smallest ion in this group. It’s the ultimate winner of the proton tug-of-war!
So, putting it all together, the order of decreasing radius (biggest to smallest) for this isoelectronic series with 10 electrons is:
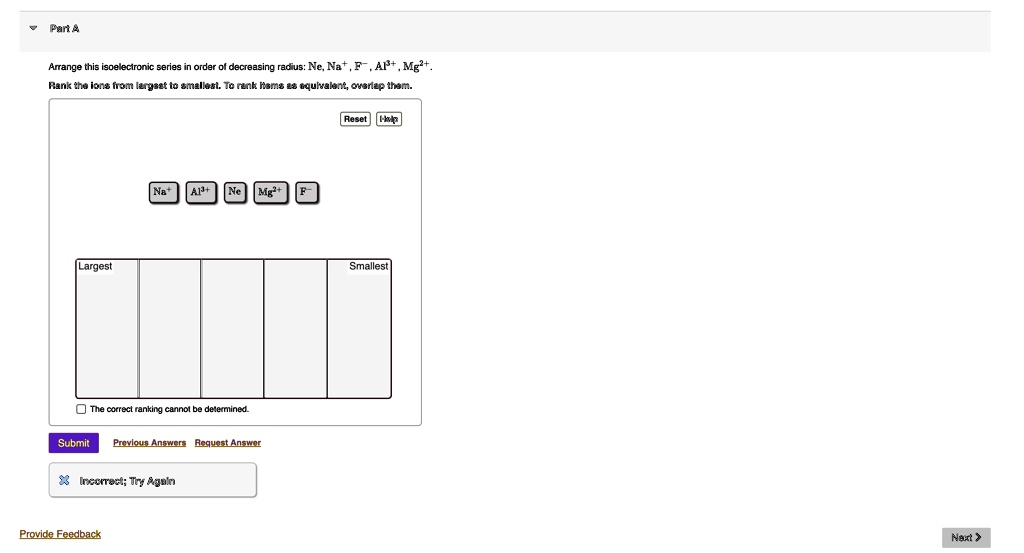
O2- > F- > Ne > Na+ > Mg2+ > Al3+
See? It’s all about that proton count! The more protons you have in that nucleus, the more power it has to cinch in those electrons, making the whole thing shrink. It’s a fundamental principle of atomic structure, and once you get the hang of it, it’s like unlocking a secret code to understanding the periodic table.
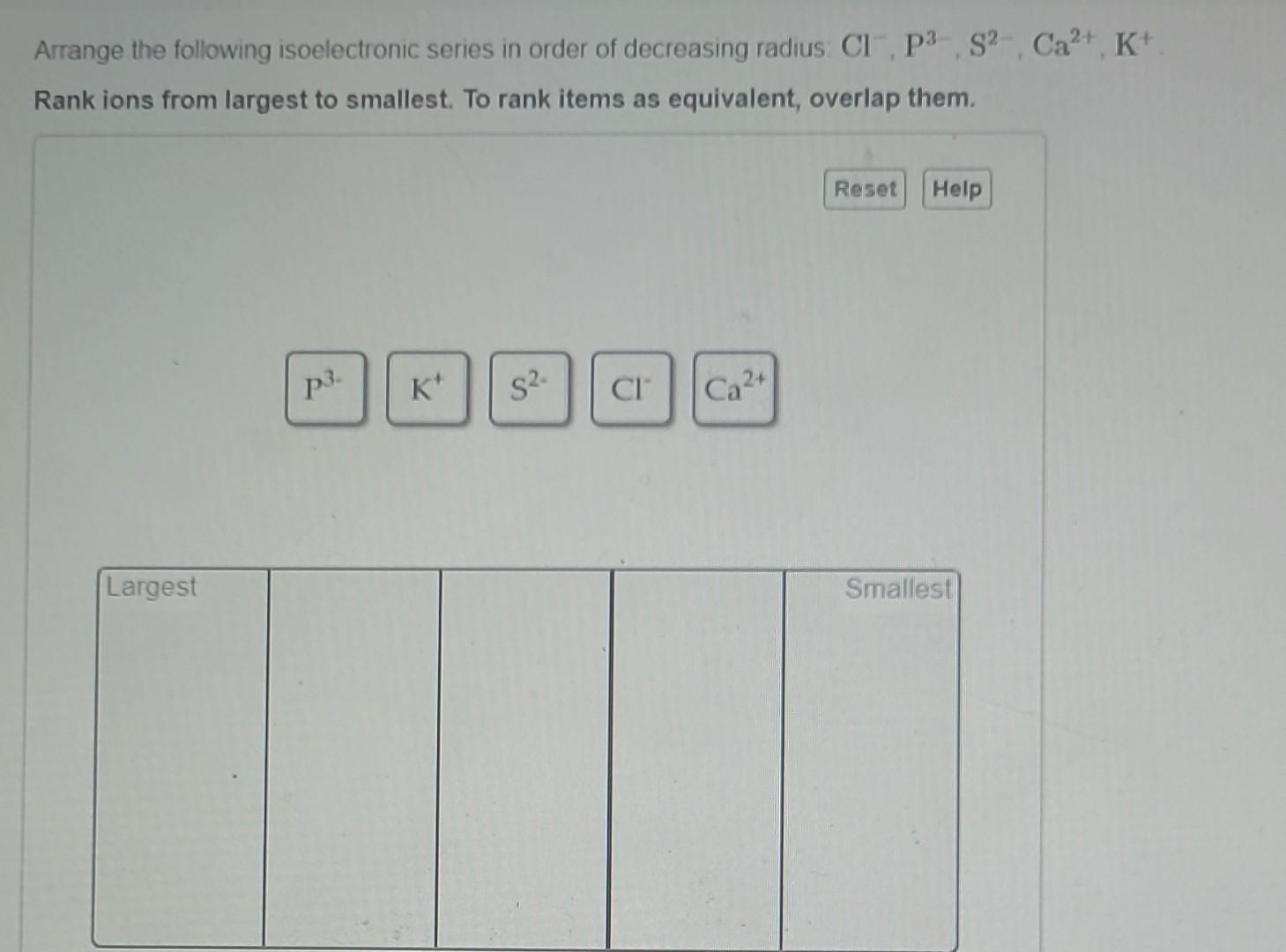
Let’s try another one, just for kicks! How about an isoelectronic series with 18 electrons? This one often includes ions of elements from the third period and beyond. Think about ions like P3-, S2-, Cl-, Ar, K+, Ca2+, and Sc3+. All these have 18 electrons.
Following our rule – fewer protons means a bigger radius – we can arrange them. Remember, we're looking for the decreasing radius, so we start with the biggest!
Phosphorus (P) has 15 protons. As P3-, it has 18 electrons. 15 protons on 18 electrons. It's pretty spread out.
Sulfur (S) has 16 protons. As S2-, it has 18 electrons. More protons than phosphorus, so it's smaller.
Chlorine (Cl) has 17 protons. As Cl-, it has 18 electrons. Even more protons, so even smaller.
Argon (Ar) has 18 protons. It's neutral with 18 electrons. The balance is perfect here.
Potassium (K) has 19 protons. As K+, it has 18 electrons. Now we're getting into where the protons outnumber the electrons, so things start shrinking.
Calcium (Ca) has 20 protons. As Ca2+, it has 18 electrons. More protons, tighter grip.
Scandium (Sc) has 21 protons. As Sc3+, it has 18 electrons. The most protons in this group, so the smallest radius.
So, the order of decreasing radius for this 18-electron series would be:

P3- > S2- > Cl- > Ar > K+ > Ca2+ > Sc3+
It's like a well-organized queue! The more positively charged protons you have at the front of the line, the more effectively they can manage the electrons behind them, keeping the whole formation compact. Pretty logical, right?
The key takeaway here is that electron count dictates membership in an isoelectronic series, but the nuclear charge (number of protons) dictates the size within that series. It's a beautiful interplay of forces that governs the tiny world of atoms.
Don't get bogged down in memorizing every single ion and its electron count. Focus on the principle! When you see a bunch of ions and atoms that could be isoelectronic, just count their electrons. If they match, then look at the protons. The higher the proton number, the smaller the radius, assuming the electron count is the same. Easy peasy, lemon squeezy!
Remember, understanding these trends helps us predict how elements will behave and interact. It's not just about memorizing facts; it's about grasping the underlying science that makes our universe tick. And that, my friends, is truly awesome.
So, the next time you're faced with an isoelectronic series, take a deep breath, count those electrons, peek at those protons, and arrange them with confidence. You’ve got this! Go forth and conquer those atomic radii challenges with a smile!
