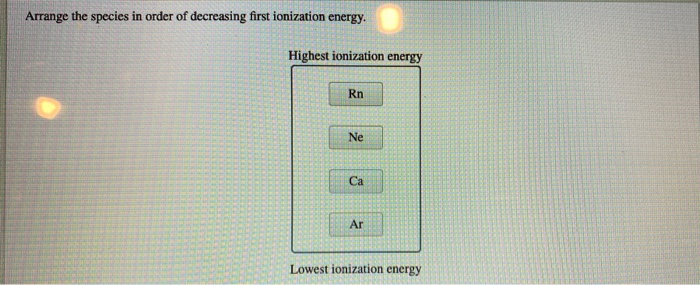
Arrange The Species In Order Of Decreasing First Ionization Energy.
Have you ever played a game of "who's the strongest"? Well, imagine a science version of that, but for tiny little things called atoms! It's called arranging species by their first ionization energy. Sounds fancy, right? But trust me, it's way more fun than it looks.
Think of it like a popularity contest for electrons. We're looking at how much of a nudge, or a push, it takes to get an electron to leave its atom. The ones that are super popular and held on tight by the atom need a really big push. Those are our champions with high ionization energy.
And then there are the electrons that are a bit more... independent. They don't need much convincing to pack their bags and go. These guys have a low ionization energy. It's like a thrilling chase to see who holds on the tightest!
This isn't just some dry science quiz, oh no. It's a puzzle! A cosmic scavenger hunt where you get to be the detective. You get to peek into the secret lives of elements and figure out their quirks. It's like understanding why your friend is always the first to grab the last cookie.
So, how do we play this game? We look at a list of elements, or "species" as the scientists like to call them. And then, we get to work ordering them. It's like sorting your favorite candies from the ones you're not so sure about.
First, we need to know our players. We've got the big names like Lithium, Beryllium, and Boron. Then there's Carbon, Nitrogen, and Oxygen. And we can't forget Fluorine and Neon! Each one has its own personality.
The rule of thumb is, as you move across a row on the periodic table, things get more exciting. The atoms get a bit clingier with their electrons. So, the ionization energy generally goes up. It's like getting closer to the ice cream truck – the anticipation builds!
Let's take a peek at that first row. We've got Lithium. It's pretty chill. Its electron is like a pet dog that's happy to go for a walk. Not too much effort to get it to leave.
Then comes Beryllium. It’s a bit more of a homebody. Its electrons are a little more settled in. You need a slightly bigger push to get one to say goodbye.
Next up is Boron. It’s starting to feel the pull of the nucleus. Its electrons are getting a tad more attached.
Carbon is next. It's got a good grip on its electrons. It’s like a teenager who's quite comfortable at home.

Nitrogen is where things get interesting. It’s like it's giving its electrons a big hug. It takes a fair amount of energy to pry one loose.
Oxygen is right after. You might think it's even stronger than Nitrogen, but here’s where the plot twist happens! Sometimes, there are little dips and bumps. It's like a roller coaster with a surprising loop!
And then we have Fluorine. This one is a real electron-lover. It's practically clinging to its electrons with all its might. It needs a massive amount of energy to let one go. It’s the ultimate electron-hugger!
Finally, we have Neon. This noble gas is like the ultimate introvert. It’s perfectly happy in its own little world. Its electron shell is full, making it super stable and very reluctant to share. It's the king of not letting go.
So, if we were to arrange these guys from least likely to let go (highest ionization energy) to most likely (lowest ionization energy), it would be a grand parade! Neon would be at the front, waving goodbye to no one.
Then comes Fluorine, holding on super tight. After that, the order might seem a bit wiggly, but the trend is generally upwards. It's like a dance with unexpected steps.
This game isn't just about memorizing numbers. It's about understanding the forces at play. It's like deciphering the secret language of the universe, one electron at a time. You start to see patterns, connections, and even a little bit of atomic drama!
And the best part? You can do this for so many different elements! You can compare elements in the same row, or even elements stacked on top of each other in columns. It’s like a never-ending puzzle book.
Imagine you have a bunch of balloons, each representing an atom. Some balloons are easily popped, while others are made of super strong material. The ionization energy is like the force needed to pop that balloon and let the air out.
Helium, for instance, is like a super-duper, reinforced balloon. It has incredibly high ionization energy. It takes a heroic effort to get one of its electrons to fly away.
Now think about Cesium. It's on the other end of the spectrum. Its electron is practically bouncing off the walls, ready to jump ship at the slightest opportunity. It has a very low ionization energy.
The fun really kicks in when you start comparing elements from different parts of the periodic table. It's like pitting a tiny, energetic chihuahua against a giant, sleepy St. Bernard. They have totally different ways of interacting with their "electrons."
Let's consider a few more players. We have Sodium, a bit like Lithium but a little further along. It’s still pretty eager to let go of an electron.
Then there's Magnesium. It’s holding on a bit tighter than Sodium, but not as tight as the non-metals. It’s a middle-ground kind of atom.
And what about Aluminum? It's somewhere between Magnesium and Silicon. It's got its own unique grip on its electrons.
Then comes Silicon. It's a bit more stubborn than Aluminum. It takes a bit more effort to convince its electrons to leave.
And Phosphorus, just before Sulfur. It's starting to show that increased hold we saw with Nitrogen.
Sulfur, next to Oxygen. It’s got a strong pull, but we know Oxygen had that funny little dip. So, comparing them directly can be a bit of a head-scratcher, which makes it all the more exciting!
Chlorine is next in line. It's a halogen, like Fluorine, so it’s very good at holding onto its electrons. It needs a good push.
And then, Argon. The noble gas counterpart to Neon. It’s even more set in its ways. Its ionization energy is sky-high. It’s the ultimate recluse.
So, let’s try putting a few of these together. If you were to arrange Sodium, Magnesium, and Aluminum, you'd see a clear trend. Sodium would be first, being the easiest to ionize. Then comes Magnesium, requiring a bit more oomph. And finally, Aluminum, holding on a bit tighter.
It's like a friendly race. Who can give up their electron the quickest? Sodium crosses the finish line with a sprint. Magnesium jogs along. And Aluminum takes its time.
Now, add in some of those elements from the row above, like Lithium and Beryllium. You'd have to remember how they fit into the grand scheme of things. It's like adding new characters to your favorite story, and you have to figure out their relationships.
The magic of arranging species by ionization energy is that it reveals the hidden order in the universe. It shows us that even the tiniest particles have rules, patterns, and personalities. It’s a way to understand why things are the way they are, from the smallest atom to the biggest star.
So, next time you hear about ionization energy, don't shy away. Think of it as a fun scientific game. A challenge to your inner detective. A chance to unlock some of the coolest secrets of the elements. It’s a delightful dive into the world of chemistry!
Go ahead, give it a try! Pick a few elements, look them up, and see if you can arrange them in order of decreasing first ionization energy. You might be surprised at how much fun you have discovering the atomic personalities! It’s a journey of discovery that’s truly out of this world!
This is where the real adventure begins! It’s like having a secret map to understanding the fundamental nature of matter. And who wouldn't want to be a part of that?
It's all about understanding those subtle forces that bind electrons to the nucleus. Some atoms are like welcoming parents, happy to let their children explore the world. Others are like strict guardians, holding their children close.
The periodic table is your playground for this game. Each element is a unique puzzle piece, and ionization energy is the key to fitting them together in a meaningful way. It's like a cosmic jigsaw puzzle that reveals the beauty of atomic structure.
So, dive in and explore! You might just find yourself captivated by the invisible world of atoms and the surprising order that governs them. It's a journey that promises wonder and a deeper appreciation for the science all around us.
It's like a treasure hunt, but the treasure is knowledge, and the map is the periodic table. And the prize? A mind-boggling understanding of how the universe works, one ionization energy at a time!
Think of it as a cosmic tug-of-war for electrons. Who wins? Who loses? It's a constant dance of attraction and repulsion that's endlessly fascinating.
The beauty of this exercise lies in its simplicity and its profound implications. You're not just memorizing facts; you're building an intuition for chemical behavior. It's like learning to read the weather by observing the clouds.
So, don't be intimidated by the fancy terms. Embrace the fun of discovery. The world of chemistry is full of exciting puzzles waiting to be solved, and arranging species by ionization energy is just the beginning of a grand adventure.
It’s a fantastic way to connect with the building blocks of everything you see. And that, my friend, is incredibly cool. It’s like having superpowers to understand the world at its most fundamental level.
