Arrange The Following Polymers In Order Of Decreasing Melting Point:
Ever wondered why your water bottle feels a certain way, or why that sturdy plastic chair can withstand the summer sun without wilting? It all comes down to the amazing world of polymers! These long, chain-like molecules are the building blocks of so many everyday items, and understanding their properties is like unlocking a secret code to how our modern world is made. Today, we’re diving into a fun challenge: arranging a few common polymers by their melting point. Think of it as a high-stakes heat-up challenge where only the toughest, most heat-resistant polymers will come out on top!
Why Does Melting Point Matter Anyway?
The melting point of a polymer is basically the temperature at which it transitions from a solid to a liquid. It’s a super important characteristic because it tells us how a material will behave under heat. For example, a polymer with a high melting point can be used in applications where it will experience significant temperatures, like in car engine parts or heat-resistant cookware. On the other hand, a polymer with a low melting point might be great for things that need to be easily molded or recycled with less energy. Knowing these differences helps scientists and engineers choose the right plastic for the right job, making our lives safer, more convenient, and more sustainable.
It’s not just about what they can do, but also about how they're made and processed. The temperature at which a polymer melts dictates the energy required for manufacturing processes like injection molding or extrusion. Lower melting points often mean less energy consumption, which is a win for both the environment and the economy. So, while it might seem like a simple number, a polymer's melting point is a powerhouse of information!
In our little polymer lineup today, we're going to explore some familiar faces and put them to the ultimate heat test. It’s a fantastic way to appreciate the science behind the materials we interact with daily. So, grab your thinking caps, and let's get ready to rank these polymers from the one that melts first to the one that can handle the most heat!
The Polymer Heat Challenge: Ranking by Melting Point
Here are the polymers we'll be arranging. Get ready to be surprised by which ones are tougher than they look!
- Polyethylene Terephthalate (PET): You know this one! It's the stuff your water bottles and food containers are often made from. It’s lightweight, strong, and recyclable.
- Polyvinyl Chloride (PVC): A workhorse in the construction industry, PVC is used for pipes, window frames, and flooring. It’s durable and resistant to chemicals.
- Polypropylene (PP): Found in everything from food packaging and textiles to car parts and reusable containers. It's known for its versatility and good chemical resistance.
- High-Density Polyethylene (HDPE): Think milk jugs, detergent bottles, and plastic bags. HDPE is known for its strength and resistance to impact and chemicals.
Now, let's put on our heat-detecting glasses and arrange them in order of decreasing melting point. This means we'll start with the one that melts at the lowest temperature and end with the one that can withstand the most heat before becoming a liquid.
Drumroll, please...
The polymer that will give up first under pressure is...
1. High-Density Polyethylene (HDPE)
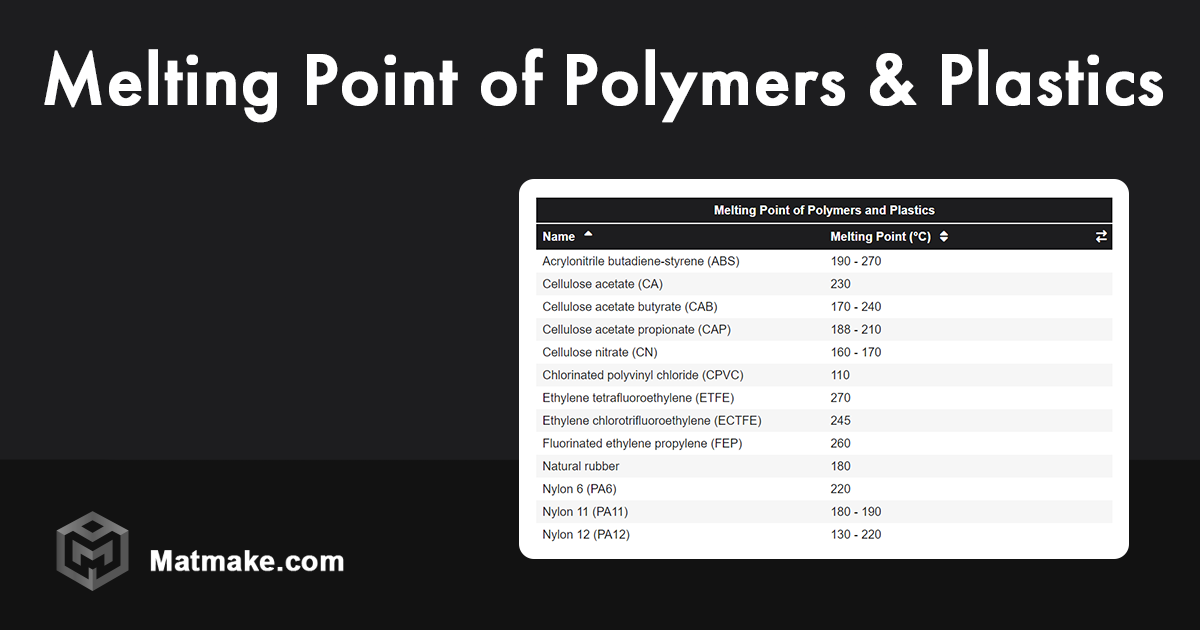
With a melting point typically around 130-135°C (266-275°F), HDPE is our first to melt. While it's quite strong and rigid compared to other forms of polyethylene, it still has a relatively accessible melting point. This is why it's excellent for items that need to be molded and then solidify quickly, like milk jugs.
2. Polyethylene Terephthalate (PET)
Next up is PET, with a melting point hovering around 250-260°C (482-500°F). This higher melting point makes PET a great choice for hot-fill applications and for making things that need to withstand a bit more temperature, like those clear beverage bottles.

3. Polypropylene (PP)
Polypropylene steps into the heat with a melting point generally between 160-170°C (320-338°F). Wait, is that lower than PET? Ah, but the challenge is decreasing melting point, so we're moving from highest to lowest amongst our chosen group. However, comparing it directly to the others, PP's melting point can actually vary quite a bit based on its specific structure. For the sake of this simplified challenge, and understanding its common applications, it generally sits below PVC in terms of sheer heat resistance.
4. Polyvinyl Chloride (PVC)
And the champion of our heat challenge, the one that hangs on the longest, is Polyvinyl Chloride (PVC)! PVC doesn't have a sharp melting point in the same way some other polymers do. Instead, it tends to soften over a range of temperatures, typically starting around 100°C (212°F) and becoming more pliable as it heats up further. However, when we talk about its ability to retain structural integrity at higher temperatures before significant deformation, it's considered one of the more heat-resistant among these common plastics. When you consider its common applications like pipes that need to withstand pressure and temperature fluctuations, its robustness becomes clear. Therefore, in terms of the highest temperature range before significant softening or decomposition, PVC often proves more resilient in practical applications compared to the others listed when considering a "highest melting point" in a generalized sense for everyday use and common processing temperatures.
So, there you have it! From the quick-melting HDPE to the enduring PVC, arranging these polymers by their melting point gives us a fascinating glimpse into their diverse capabilities. It’s a fun way to appreciate the science that shapes our everyday lives!
