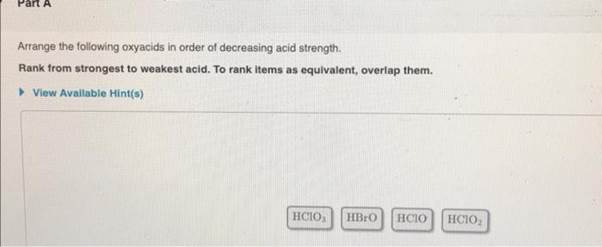
Arrange The Following Oxyacids In Order Of Decreasing Acid Strength.
Ever wondered why some acids are super sour and others are more like a gentle nudge? It's a question that pops up in science class, in the kitchen, and even when you're just curious about the world around you. Understanding acid strength isn't just for chemists; it's a fascinating peek into how different substances behave and interact. Think of it like a superhero ranking – who's the strongest, who's the mildest? That's what we're diving into today, but instead of capes and superpowers, we're looking at the amazing world of oxyacids.
The "Why" Behind the Pucker
So, what's the big deal about acid strength? Well, it's incredibly useful! For starters, it helps us predict how chemicals will react. If you're baking, knowing if an acid is strong or weak can affect how your leavening agents work. In medicine, understanding acid strength is crucial for formulating treatments that are effective but don't harm the body. And for environmental science, it's key to understanding how substances in the soil and water behave. Plus, let's be honest, there's a certain thrill in deciphering scientific puzzles, and ranking these oxyacids is a prime example. It’s like solving a delicious chemistry riddle!
The fun part comes from seeing how a few subtle differences in their structure lead to big differences in their behavior. It’s a beautiful demonstration of how the molecular world dictates the macroscopic world we experience. We’re not just memorizing facts; we’re uncovering the logic of nature. This topic is popular because it’s a gateway to understanding a vast range of chemical phenomena, from the tang of your favorite citrus fruit to the industrial processes that shape our modern lives.
The Oxyacid All-Stars
Let's meet our contenders! We're focusing on a special group of acids called oxyacids. These are acids that contain oxygen atoms bonded to a central atom, which is often a non-metal. Think of them as tiny molecular structures, each with its own personality and power level. The strength of an oxyacid is all about its ability to give away a proton (a positively charged hydrogen ion, H+). The easier it is for the acid to release that proton, the stronger it is. And what makes it easier or harder? You guessed it – the atoms involved and how they're arranged!
To understand their strength, we need to look at a few key factors. One is the number of oxygen atoms attached to the central atom. Generally, the more oxygen atoms there are, the stronger the acid. This is because the extra oxygen atoms are really good at pulling electron density away from the bond between the central atom and the hydrogen, making that hydrogen more eager to leave. Another factor is the electronegativity of the central atom. A more electronegative central atom will also pull electron density, making the acid stronger. It’s all about the tug-of-war for electrons!

The Grand Ranking
Now for the exciting part – putting them in order! This is where we see the practical application of those structural insights. Imagine you have a lineup of these oxyacids, and you need to arrange them from the most powerful (the one that readily hands over its protons) to the least powerful. It's like a friendly competition where we're measuring their acidic "oomph."
The goal is to order them by decreasing acid strength. This means starting with the acid that is most willing to donate a proton and ending with the one that is least willing. It’s a clear-cut way to compare their chemical personalities.
Arrange the following in decreasing order of acid strength - YouTube
Let’s consider a common set of oxyacids to illustrate this. We’ll look at acids formed by elements in the same period or group to highlight the specific factors at play. For instance, comparing oxyacids of halogens like HClO, HClO2, HClO3, and HClO4 (hypochlorous acid, chlorous acid, chloric acid, and perchloric acid) is a classic example. Here, the number of oxygen atoms is the primary driver of strength. Perchloric acid (HClO4), with four oxygen atoms, is a very strong acid, while hypochlorous acid (HClO), with only one, is much weaker.
Similarly, if we look at oxyacids of elements in the same group but with the same number of oxygen atoms, like H2SO4 (sulfuric acid) and H2SeO4 (selenic acid), the electronegativity of the central atom comes into play. Sulfur is more electronegative than selenium, making sulfuric acid a stronger acid than selenic acid.
So, when you're asked to arrange oxyacids in order of decreasing acid strength, you're essentially applying these rules of thumb. You're looking at the central atom, counting the oxygen atoms, and considering electronegativity. It's a process of analytical reasoning that leads to a definitive order. This skill is incredibly valuable, allowing you to predict reactions, understand chemical properties, and even make informed decisions in everyday life. It’s a satisfying way to bring order to the chemical world!

