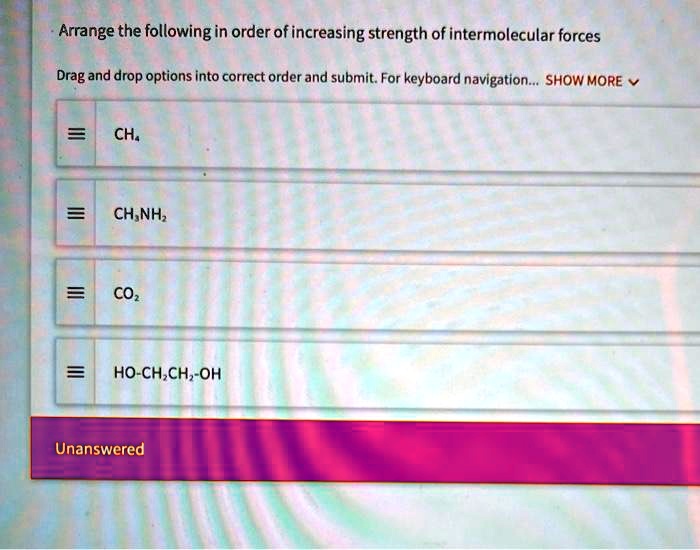
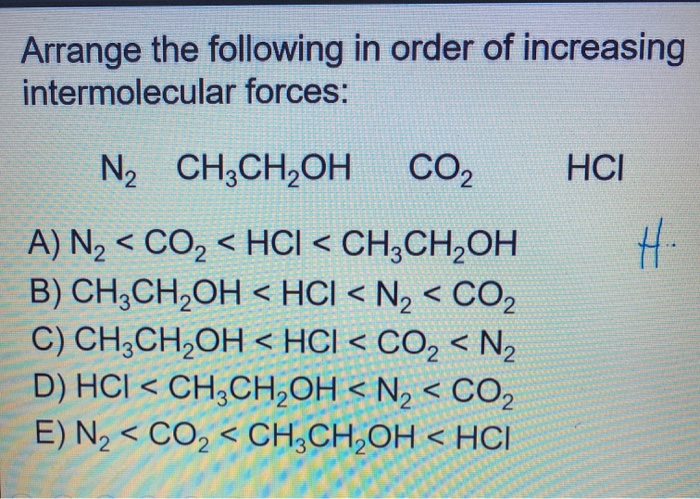
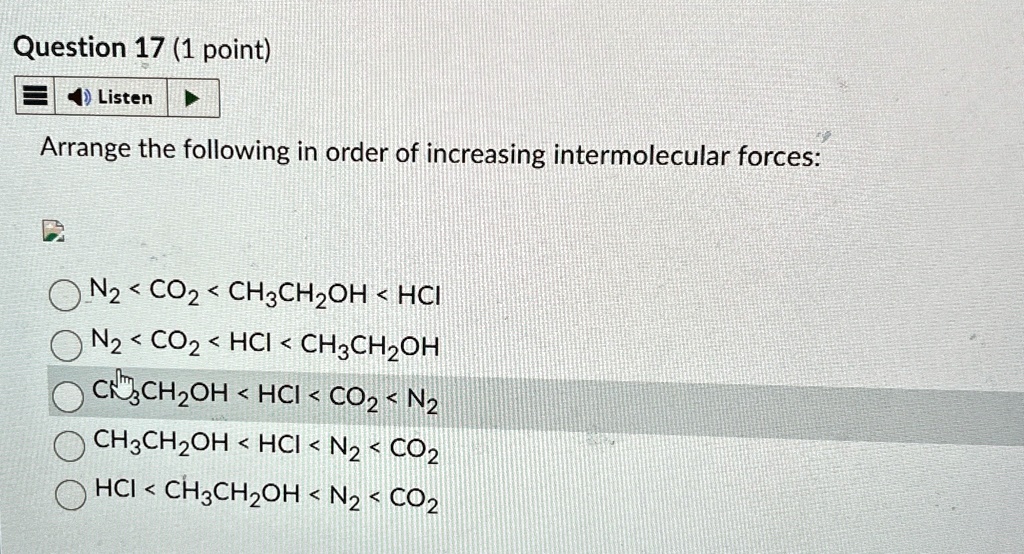
Arrange The Following In Order Of Increasing Intermolecular Forces
Get ready to embark on a super-duper, hilariously simple adventure into the invisible world of how stuff sticks together! We're going to talk about something called intermolecular forces. Now, don't let that fancy name scare you. Think of it like this: it's the secret handshake that molecules give each other to decide if they want to be buddies or, well, let's just say "acquaintances." Some molecules are super clingy, practically Siamese twins, while others are like those people who politely nod at you in the hallway and then disappear. We're going to arrange a few common substances from "barely know ya" to "super glue besties" based on how strong these invisible hugs are.
Imagine you've got a party, and you're trying to get your friends to form a circle. Some friends might just drift around the edges, happy to be in the same room but not really joining the main event. Others might grab hands immediately, forming a tight little huddle. That, my friends, is the essence of intermolecular forces! We're ranking our substances from the most "loner" to the most "group hug extraordinaire."
First up, we have helium. Oh, helium! This gas is so chill, so carefree, it’s like the ultimate hermit crab of the molecular world. Helium atoms are practically waving at each other from across a vast, empty ballroom. They’re so far apart, and honestly, they’ve got their own things going on. The forces between helium atoms are so weak, they’re practically non-existent. It’s like trying to make friends with a ghost – a very polite, very aloof ghost. They just don't feel the need for much interaction. They’re happy floating around solo, doing their own atomic thing. You could practically will them apart if you tried hard enough. They’re the ultimate individualists. So, when we talk about the weakest of the weak, the absolute kings and queens of social distancing in the molecular kingdom, we’re talking about helium.
Next in line, we're stepping it up just a tiny, tiny notch. Think of methane. Now, methane is a gas too, like helium, but it’s a little more social. Imagine our helium friends have decided to maybe, just maybe, hold hands for a fleeting second before letting go again. Methane molecules are a bit like that. They're not exactly besties, but they’re not complete strangers either. They have a little bit more of a nudge-nudge, wink-wink relationship. It's like they’re at a party, and they might brush past each other, give a slight nod, and then continue on their merry way. The forces here are still pretty weak, mind you. It’s like a polite handshake that lasts for about a nanosecond. They’re not going to form a human pyramid or anything. They’re still very much in the "acquaintance zone." It’s a step up from pure, unadulterated aloofness, but we’re still a long way from anything resembling a strong bond.
Now, let’s pump up the excitement (and the forces!) a bit more. Get ready for water! Ah, water. The lifeblood of everything! Water molecules are like those super enthusiastic friends who are always ready for a group hug. They absolutely love sticking together. Think about how water forms puddles, how it clings to your skin after a shower, or how it forms those beautiful, intricate snowflakes. That's all thanks to the fact that water molecules are total lovebugs. They’ve got these special little attractions, like tiny magnets, pulling them towards each other. It’s like they’re all holding hands, arms around each other’s shoulders, forming a happy, cohesive bunch. This is why water is a liquid at room temperature, instead of a gas like helium and methane. Those molecules are really enjoying each other's company. They’re not just drifting; they're actively choosing to stay close. It's a much stronger connection than a fleeting nod or a nanosecond handshake. This is the kind of force that makes things stay put and have substance.

And finally, the ultimate cling-ons, the undisputed champions of sticking together: ice. Now, this might seem a bit sneaky, because ice is just frozen water. But here’s the twist! When water freezes into ice, those water molecules that were happily sloshing around in their group hugs decide to organize themselves into an even more rigid, more structured, and therefore even stronger arrangement. Think of it as the ultimate bonding experience. They’ve gone from a lively dance party to a perfectly choreographed ballet. The forces between the water molecules in ice are at their absolute peak. They're locked in place, holding on with all their might. It’s like they’ve formed a giant, interlocking jigsaw puzzle of pure molecular affection. If intermolecular forces were a competition for who can cling the hardest, ice would be wearing the gold medal. It’s the most tightly bound of the bunch, the ultimate testament to the power of molecules deciding they really, really don't want to let go of each other. So, from the loneliest atom to the most tightly knit crystal, we've journeyed through the fascinating world of invisible molecular cuddles!
So, to recap our thrilling journey from "meh" to "OMG, never let go!":Helium (the ultimate loner)
SOLVED: Arrange the following in order of increasing strength ofMethane (polite nod)
Water (happy group hug)
Solved Arrange the following in order of increasing | Chegg.comIce (super-duper rigid, unbreakable bond!)
Isn't science fun? We just ranked things by how much they want to be friends, and it all comes down to these tiny, invisible forces. Who knew molecules could be such social creatures (or not!). Keep looking around you, and you'll see these forces at play everywhere!