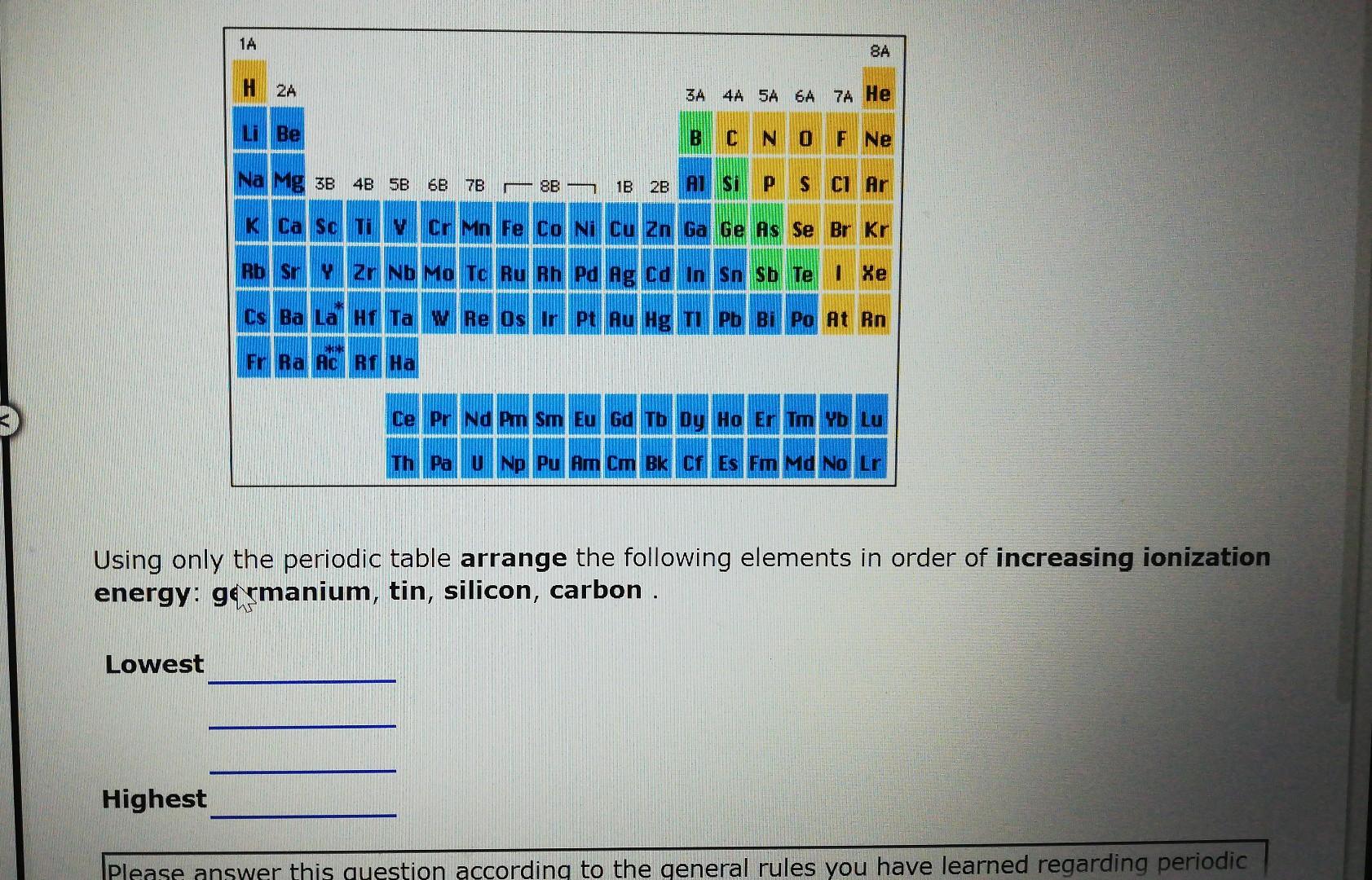
Arrange The Following Elements In Order Of Increasing Ionization Energy
Imagine you're at a super exclusive party, a real "who's who" of the universe's building blocks. Everyone's trying to get in, but there's a catch: they need to shed a little something extra to be accepted. We're talking about those stubborn little electrons, those tiny charges that orbit around the center of an atom. It's like they're holding onto their identity with all their might!
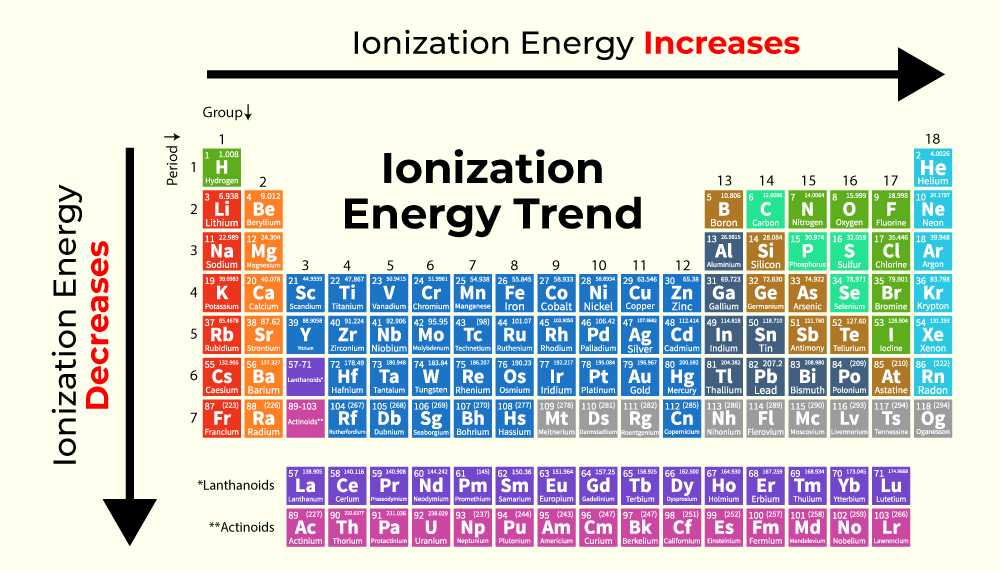
This party is all about ionization energy, which is just a fancy way of saying how much effort, or energy, it takes to convince an electron to leave its atom home and go wander off on its own. Think of it as the atom's "leaving home" fee. Some atoms are practically giving away electrons, while others are fiercely protective, demanding a hefty bribe.
Let's meet some of our party guests. First up, we have Sodium. Now, Sodium is a bit of a drama queen. It's got this one extra electron, sitting all by itself, just begging to be set free. It's practically waving a little flag saying, "Take me, please! I'm so lonely!"
So, Sodium is ready to pay a very, very small fee to get rid of that lone electron. It's the easiest one to convince, the first one through the door, barely breaking a sweat. It’s like it’s been waiting for this moment its whole atomic existence.
Next in line, we have Aluminum. Aluminum is a bit more complex. It's got more electrons, and they're more organized. It's not just one lone ranger; it's a small group.
While Aluminum still wants to shed some electrons to feel more stable, it's a bit more attached. It's like it needs a slightly bigger push, a slightly higher fee. It’s not as eager as Sodium, but it's definitely looking for an exit strategy.
Then comes Phosphorus. Phosphorus is a bit of a pack animal. It likes to keep its electron buddies close. It’s more about fitting in and stability within its own group.
To get an electron to leave Phosphorus, you need to put in a bit more effort. It's not as straightforward as Sodium's lonely electron, and it’s not as organized as Aluminum's little cluster. It's somewhere in the middle, a bit more reluctant.
Now, let's talk about Sulfur. Sulfur is even more of a team player than Phosphorus. It's got a strong sense of community amongst its electrons.
Sulfur is really good at holding onto its electrons. It requires a significant amount of energy to pry one away. It's like trying to get a child to give up their favorite toy; it's going to take some serious convincing!
And finally, we have Chlorine. Chlorine is the ultimate electron hoarder. It's practically glued to its electrons, almost daring you to try and take one away.
Chlorine has a really, really high ionization energy. It’s the one who slams the door shut and puts up a "No Vacancy" sign. You'll need to bring your A-game, your highest energy offerings, to even get a whisper of a chance to remove an electron from Chlorine.
So, if we were to line them all up at the party entrance, based on how easily they're willing to let an electron go, it would look like this: First, the super-eager Sodium, practically handing over its electron with a smile and a wave. Then, a bit more persuasion is needed for Aluminum, which is willing but not thrilled to part with an electron.
Next up is Phosphorus, requiring a bit more of a convincing argument. Then comes Sulfur, who's really digging its heels in, needing a good amount of coaxing. And finally, bringing up the rear, is the incredibly reluctant Chlorine, demanding the highest "leaving home" fee of all.
It's like a game of "Keep Away" with electrons! Some atoms are terrible at it, others are absolute pros. And this little dance of electron-dodging actually explains a lot about how different elements behave and interact with each other.

Think about how they might react in a chemical reaction. The ones with low ionization energy are like magnets for reactions, happy to give away electrons and form new bonds. The ones with high ionization energy are more aloof, preferring to observe from a distance, or perhaps trying to snatch electrons from others!
It's a whole universe of atomic personalities, each with their own unique energy level for letting go. And it all comes down to this simple idea: how much energy does it take to make them say goodbye to an electron?
It’s fascinating to think that even something as tiny as an electron can have such a profound impact on the grand scheme of things. These little guys are the unsung heroes of chemistry, and their willingness (or unwillingness) to leave home shapes everything we see around us.
So, the next time you encounter these elements, whether it's in a science class or just a passing thought, remember their little electron-shedding party. It’s a quirky, energetic, and surprisingly heartwarming tale of atomic ambition and electron attachment.

And the order, from the easiest to let go to the most stubbornly attached, is a little story in itself. It’s a spectrum of willingness, a measure of atomic resolve.
It's a testament to how even in the most fundamental building blocks of our universe, there's a dynamic interplay of forces and a whole lot of personality!
So, let's raise a toast to ionization energy, the invisible force that dictates so much about the world we live in. It’s a fun way to understand the characters of the periodic table!
Each element, with its unique energy signature, contributes to the grand symphony of chemical reactions that make our world so vibrant and diverse. It’s a beautiful, energetic ballet.
